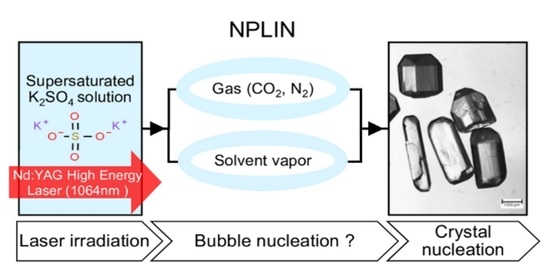
Potassium Sulfate: A New Candidate to Explore Non-Photochemical Laser-Induced Nucleation Mechanisms
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Metastable Solutions of K2SO4 in Water
2.2. NPLIN Setup
2.3. NPLIN Experiments
2.4. Preparation of the K2SO4 Solutions Saturated with Different Gas
3. Results and Discussion
3.1. Influence of Supersaturation on the Nucleation Kinetics and the Probability of Nucleation
3.2. Influence of the Number of Pulses and Intensity
3.3. Influence of the Laser Polarization
3.4. Influence of Gas Composition and Nature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Garetz, B.A.; Aber, J.E.; Goddard, N.L.; Young, R.G.; Myerson, A.S. Nonphotochemical, Polarization-Dependent, Laser-Induced Nucleation in Supersaturated Aqueous Urea Solutions. Phys. Rev. Lett. 1996, 77, 3475. [Google Scholar] [CrossRef] [Green Version]
- Garetz, B.A.; Matic, J.; Myerson, A.S. Polarization Switching of Crystal Structure in the Nonphotochemical Light-Induced Nucleation of Supersaturated Aqueous Glycine Solutions. Phys. Rev. Lett. 2002, 89, 175501. [Google Scholar] [CrossRef]
- Sun, X.; Garetz, B.A.; Myerson, A.S. Polarization Switching of Crystal Structure in the Nonphotochemical Laser-Induced Nucleation of Supersaturated Aqueous l-Histidine. Cryst. Growth Des. 2008, 8, 1720–1722. [Google Scholar] [CrossRef]
- Wang, S.; Wang, S.; Jiang, L.; Wang, M.; Wei, Y.; Sun, J.; Zhan, S.; Li, X.; Qu, L. Polymorph-Controlled Crystallization of Acetaminophen through Femtosecond Laser Irradiation. Cryst. Growth Des. 2019, 19, 3265–3271. [Google Scholar] [CrossRef]
- Li, W.; Ikni, A.; Scouflaire, P.; Shi, X.; El Hassan, N.; Gémeiner, P.; Gillet, J.-M.; Spasojević-de Biré, A. Non-Photochemical Laser-Induced Nucleation of Sulfathiazole in a Water/Ethanol Mixture. Cryst. Growth Des. 2016, 16, 2514–2526. [Google Scholar] [CrossRef]
- Alexander, A.J.; Camp, P.J. Single Pulse, Single Crystal Laser-Induced Nucleation of Potassium Chloride. Cryst. Growth Des. 2009, 9, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.R.; Mackenzie, A.M.; Alexander, A.J. Role of Impurity Nanoparticles in Laser-Induced Nucleation of Ammonium Chloride. Cryst. Growth Des. 2016, 16, 6790–6796. [Google Scholar] [CrossRef]
- Barber, E.R.; Kinney, N.L.H.; Alexander, A.J. Pulsed Laser-Induced Nucleation of Sodium Chlorate at High Energy Densities. Cryst. Growth Des. 2019, 19, 7106–7111. [Google Scholar] [CrossRef]
- Jacob, J.A.; Sorgues, S.; Dazzi, A.; Mostafavi, M.; Belloni, J. Homogeneous Nucleation-Growth Dynamics Induced by Single Laser Pulse in Supersaturated Solutions. Cryst. Growth Des. 2012, 12, 5980–5985. [Google Scholar] [CrossRef]
- Soare, A.; Dijkink, R.; Pascual, M.R.; Sun, C.; Cains, P.W.; Lohse, D.; Stankiewicz, A.I.; Kramer, H.J.M. Crystal Nucleation by Laser-Induced Cavitation. Cryst. Growth Des. 2011, 11, 2311–2316. [Google Scholar] [CrossRef] [Green Version]
- Lee, I.S.; Evans, J.M.B.; Erdemir, D.; Lee, A.Y.; Garetz, B.A.; Myerson, A.S. Nonphotochemical Laser Induced Nucleation of Hen Egg White Lysozyme Crystals. Cryst. Growth Des. 2008, 8, 4255–4261. [Google Scholar] [CrossRef]
- Yoshikawa, H.Y.; Murai, R.; Sugiyama, S.; Sazaki, G.; Kitatani, T.; Takahashi, Y.; Adachi, H.; Matsumura, H.; Murakami, S.; Inoue, T.; et al. Femtosecond Laser-Induced Nucleation of Protein in Agarose Gel. J. Cryst. Growth 2009, 311, 956–959. [Google Scholar] [CrossRef]
- Murai, R.; Yoshikawa, H.Y.; Hasenaka, H.; Takahashi, Y.; Maruyama, M.; Sugiyama, S.; Adachi, H.; Takano, K.; Matsumura, H.; Murakami, S.; et al. Influence of Energy and Wavelength on Femtosecond Laser-Induced Nucleation of Protein. Chem. Phys. Lett. 2011, 510, 139–142. [Google Scholar] [CrossRef]
- Zaccaro, J.; Matic, J.; Myerson, A.S.; Garetz, B.A. Nonphotochemical, Laser-Induced Nucleation of Supersaturated Aqueous Glycine Produces Unexpected γ-Polymorph. Cryst. Growth Des. 2001, 1, 5–8. [Google Scholar] [CrossRef]
- Matic, J.; Sun, X.; Garetz, B.A.; Myerson, A.S. Intensity, Wavelength, and Polarization Dependence of Nonphotochemical Laser-Induced Nucleation in Supersaturated Aqueous Urea Solutions. Cryst. Growth Des. 2005, 5, 1565–1567. [Google Scholar] [CrossRef]
- Sun, X.; Garetz, B.A.; Myerson, A.S. Supersaturation and Polarization Dependence of Polymorph Control in the Nonphotochemical Laser-Induced Nucleation (NPLIN) of Aqueous Glycine Solutions. Cryst. Growth Des. 2006, 6, 684–689. [Google Scholar] [CrossRef]
- Clair, B.; Ikni, A.; Li, W.; Scouflaire, P.; Quemener, V.; Spasojević-de Biré, A. A New Experimental Setup for High-Throughput Controlled Non-Photochemical Laser-Induced Nucleation: Application to Glycine Crystallization. J. Appl. Cryst. 2014, 47, 1252–1260. [Google Scholar] [CrossRef]
- Liu, Y.; van den Berg, M.H.; Alexander, A.J. Supersaturation Dependence of Glycine Polymorphism Using Laser-Induced Nucleation, Sonocrystallization and Nucleation by Mechanical Shock. Phys. Chem. Chem. Phys. 2017, 19, 19386–19392. [Google Scholar] [CrossRef] [Green Version]
- Irimia, D.; Jose Shirley, J.; Garg, A.S.; Nijland, D.P.A.; van der Heijden, A.E.D.M.; Kramer, H.J.M.; Eral, H.B. Influence of Laser Parameters and Experimental Conditions on Nonphotochemical Laser-Induced Nucleation of Glycine Polymorphs. Cryst. Growth Des. 2020, 21, 631–641. [Google Scholar] [CrossRef]
- Liu, Y.; Ward, M.R.; Alexander, A.J. Polarization Independence of Laser-Induced Nucleation in Supersaturated Aqueous Urea Solutions. Phys. Chem. Chem. Phys. 2017, 19, 3464–3467. [Google Scholar] [CrossRef] [Green Version]
- Knott, B.C.; Doherty, M.F.; Peters, B. A Simulation Test of the Optical Kerr Mechanism for Laser-Induced Nucleation. J. Chem. Phys. 2011, 134, 154501. [Google Scholar] [CrossRef]
- Knott, B.C.; LaRue, J.L.; Wodtke, A.M.; Doherty, M.F.; Peters, B. Communication: Bubbles, Crystals, and Laser-Induced Nucleation. J. Chem. Phys. 2011, 134, 171102. [Google Scholar] [CrossRef] [PubMed]
- Rey-García, F.; Sieira, B.J.; Bao-Varela, C.; Leis, J.R.; Angurel, L.A.; Quintana, J.B.; Rodil, R.; de la Fuente, G.F. Can UV-C Laser Pulsed Irradiation Be Used for the Removal of Organic Micropollutants from Water? Case Study with Ibuprofen. Sci. Total Environ. 2020, 742, 140507. [Google Scholar] [CrossRef] [PubMed]
- Javid, N.; Kendall, T.; Burns, I.S.; Sefcik, J. Filtration Suppresses Laser-Induced Nucleation of Glycine in Aqueous Solutions. Cryst. Growth Des. 2016, 16, 4196–4202. [Google Scholar] [CrossRef] [Green Version]
- Ward, M.R.; Jamieson, W.J.; Leckey, C.A.; Alexander, A.J. Laser-Induced Nucleation of Carbon Dioxide Bubbles. J. Chem. Phys. 2015, 142, 144501. [Google Scholar] [CrossRef] [Green Version]
- Mirsaleh-Kohan, N.; Fischer, A.; Graves, B.; Bolorizadeh, M.; Kondepudi, D.; Compton, R.N. Laser Shock Wave Induced Crystallization. Cryst. Growth Des. 2017, 17, 576–581. [Google Scholar] [CrossRef]
- Kacker, R.; Dhingra, S.; Irimia, D.; Ghatkesar, M.K.; Stankiewicz, A.; Kramer, H.J.M.; Eral, H.B. Multiparameter Investigation of Laser-Induced Nucleation of Supersaturated Aqueous KCl Solutions. Cryst. Growth Des. 2018, 18, 312–317. [Google Scholar] [CrossRef]
- del Río-Sancho, S.; Pan Delgado, D.; de la Fuente, G.F.; García-Caballero, T.; Taboada-Suárez, A.; Csaba, N.; Bao-Varela, C.; José Alonso, M. Laser-Induced Transient Skin Disruption to Enhance Cutaneous Drug Delivery. Eur. J. Pharm. Biopharm. 2020, 156, 165–175. [Google Scholar] [CrossRef] [PubMed]
- McGinnety, J.A. Redetermination of the Structures of Potassium Sulphate and Potassium Chromate: The Effect of Electrostatic Crystal Forces upon Observed Bond Lengths. Acta Cryst. B 1972, 28, 2845–2852. [Google Scholar] [CrossRef]
- Arnold, H.; Kurtz, W.; Richter-Zlnnius, A.; Bethke, J.; Heger, G. The Phase Transition of K2SO4 at about 850 K. Acta Crystallogr. Sect. B 1981, 37, 1643–1651. [Google Scholar] [CrossRef]
- Mydlarz, J.; Jones, A.G. Potassium Sulfate Water-Alcohols Systems: Composition and Density of Saturated Solutions. J. Chem. Eng. Data 1990, 35, 214–216. [Google Scholar] [CrossRef]
- Mydlarz, J.; Jones, A.G.; Millan, A. Solubility and Density Isotherms for Potassium Sulfate-Water-2-Propanol. J. Chem. Eng. Data 1989, 34, 124–126. [Google Scholar] [CrossRef]
- Polyanskiy, M.N. Refractive Index Database. Available online: https://refractiveindex.info. (accessed on 15 July 2021).
- Jiang, S.; ter Horst, J.H. Crystal Nucleation Rates from Probability Distributions of Induction Times. Cryst. Growth Des. 2011, 11, 256–261. [Google Scholar] [CrossRef]
- Hua, T.; Gowayed, O.; Grey-Stewart, D.; Garetz, B.A.; Hartman, R.L. Microfluidic Laser-Induced Nucleation of Supersaturated Aqueous KCl Solutions. Cryst. Growth Des. 2019, 19, 3491–3497. [Google Scholar] [CrossRef]
- Matviiv, R.B.; Rudysh, M.Y.; Stadnyk, V.Y.; Fedorchuk, A.O.; Shchepanskyi, P.A.; Brezvin, R.S.; Khyzhun, O.Y. Structure, Refractive and Electronic Properties of K2SO4: Cu2+ (3%) Crystals. Curr. Appl. Phys. 2021, 21, 80–88. [Google Scholar] [CrossRef]
- Wilhelm, E.; Battino, R.; Wilcock, R.J. Low-Pressure Solubility of Gases in Liquid Water. Chem. Rev. 1977, 77, 219–262. [Google Scholar] [CrossRef]
- Lee, J.I.; Yim, B.-S.; Kim, J.-M. Effect of Dissolved-Gas Concentration on Bulk Nanobubbles Generation Using Ultrasonication. Sci. Rep. 2020, 10, 18816. [Google Scholar] [CrossRef]




| Np | Ep (mJ) | Induction Time (min) | Probability of Nucleation (%) (t = 1.5 h) | Number of Crystals Per Vial (min–max) |
|---|---|---|---|---|
| 1 | 18 | 60 | 40 | 1–4 |
| 101 | 18 | 60 | 80 | 1–8 |
| 1 | 85.6 | 30 | 100 | 3–6 |
| 101 | 85.6 | 30 | 100 | >30 |
| Polarization | Induction Time (min) | Probability of Nucleation % (t = 1.5 h) | Number of Crystals Per Vial (min–max) |
|---|---|---|---|
| LP | 30 | 100 | 3–6 |
| RCP | 30 | 100 | 2–6 |
| LCP | 30 | 100 | 2–7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Briard, M.; Brandel, C.; Morin-Grognet, S.; Coquerel, G.; Dupray, V. Potassium Sulfate: A New Candidate to Explore Non-Photochemical Laser-Induced Nucleation Mechanisms. Crystals 2021, 11, 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121571
Briard M, Brandel C, Morin-Grognet S, Coquerel G, Dupray V. Potassium Sulfate: A New Candidate to Explore Non-Photochemical Laser-Induced Nucleation Mechanisms. Crystals. 2021; 11(12):1571. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121571
Chicago/Turabian StyleBriard, Mélody, Clément Brandel, Sandrine Morin-Grognet, Gérard Coquerel, and Valérie Dupray. 2021. "Potassium Sulfate: A New Candidate to Explore Non-Photochemical Laser-Induced Nucleation Mechanisms" Crystals 11, no. 12: 1571. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11121571