Numerical Simulation of Ammonothermal Crystal Growth of GaN—Current State, Challenges, and Prospects
Abstract
:1. Introduction
2. Functionality of the Ammonothermal GaN Growth Process
3. Simulations of Fluid Flow and Temperature Field
3.1. Simulation Domain and Geometry
3.2. Axisymmetric 2D Calculations versus 3D Calculations
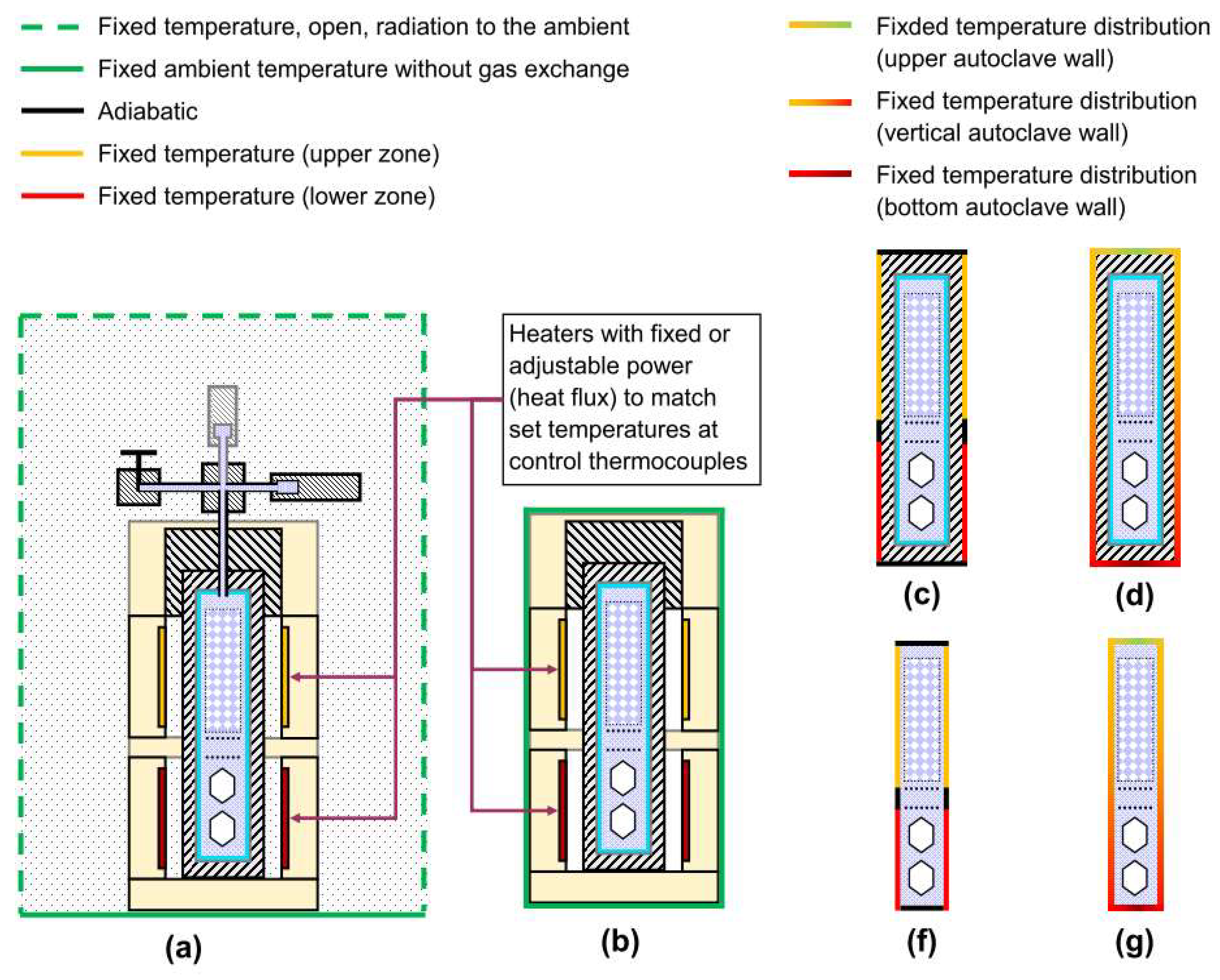
3.3. Boundary Conditions
3.4. Physics and Models Thereof
3.5. Discretization in Space and Time
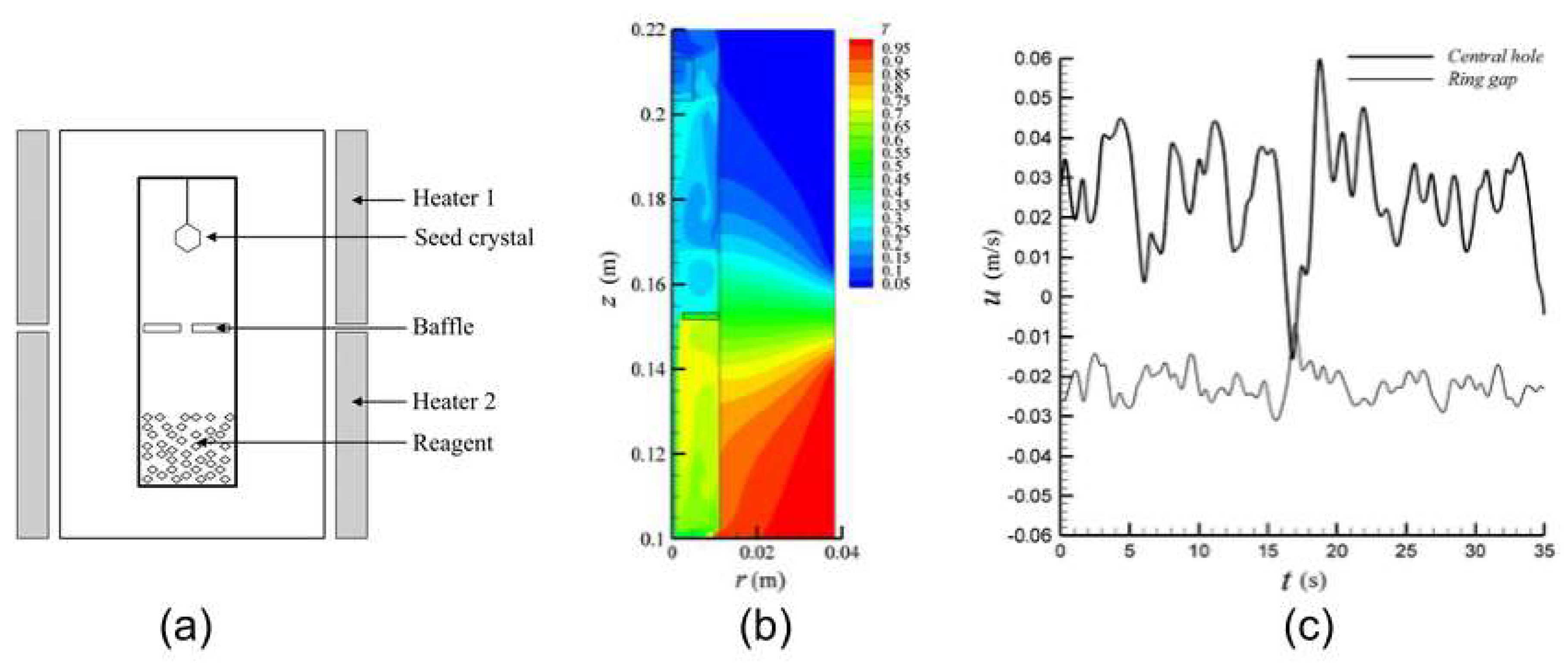
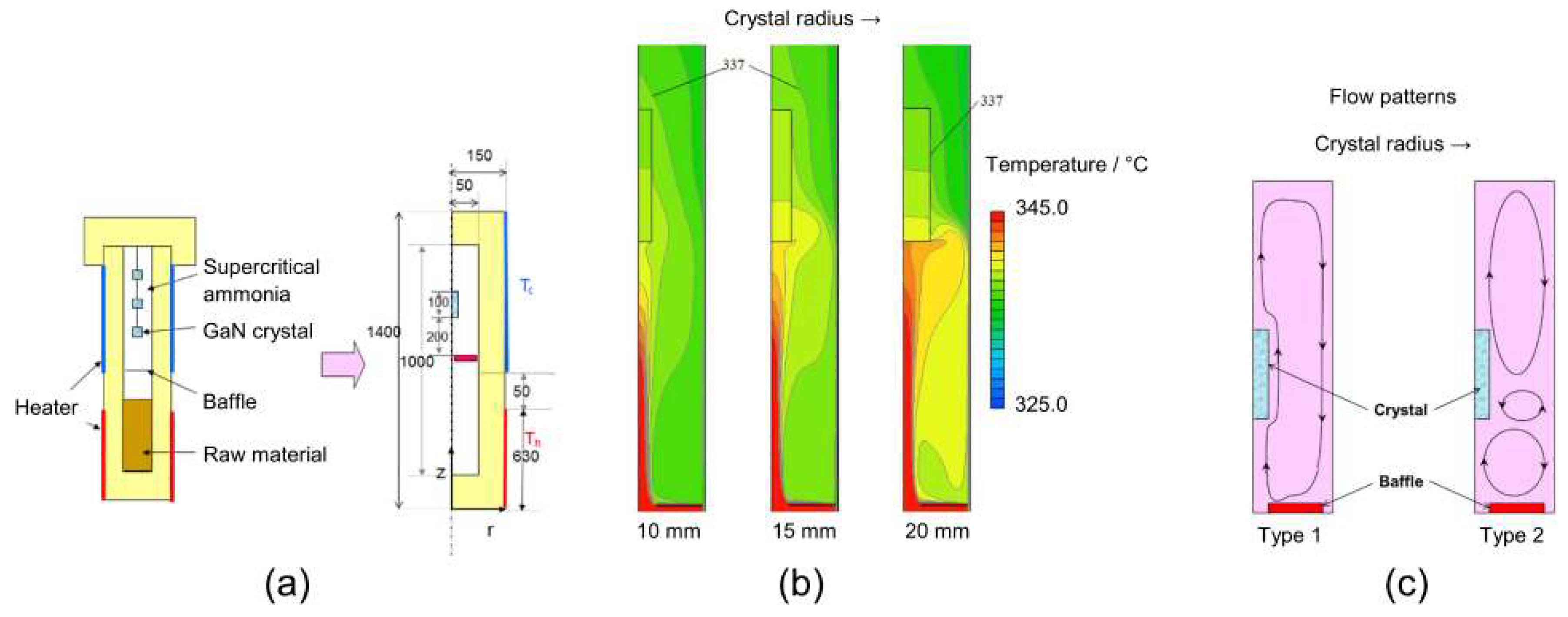
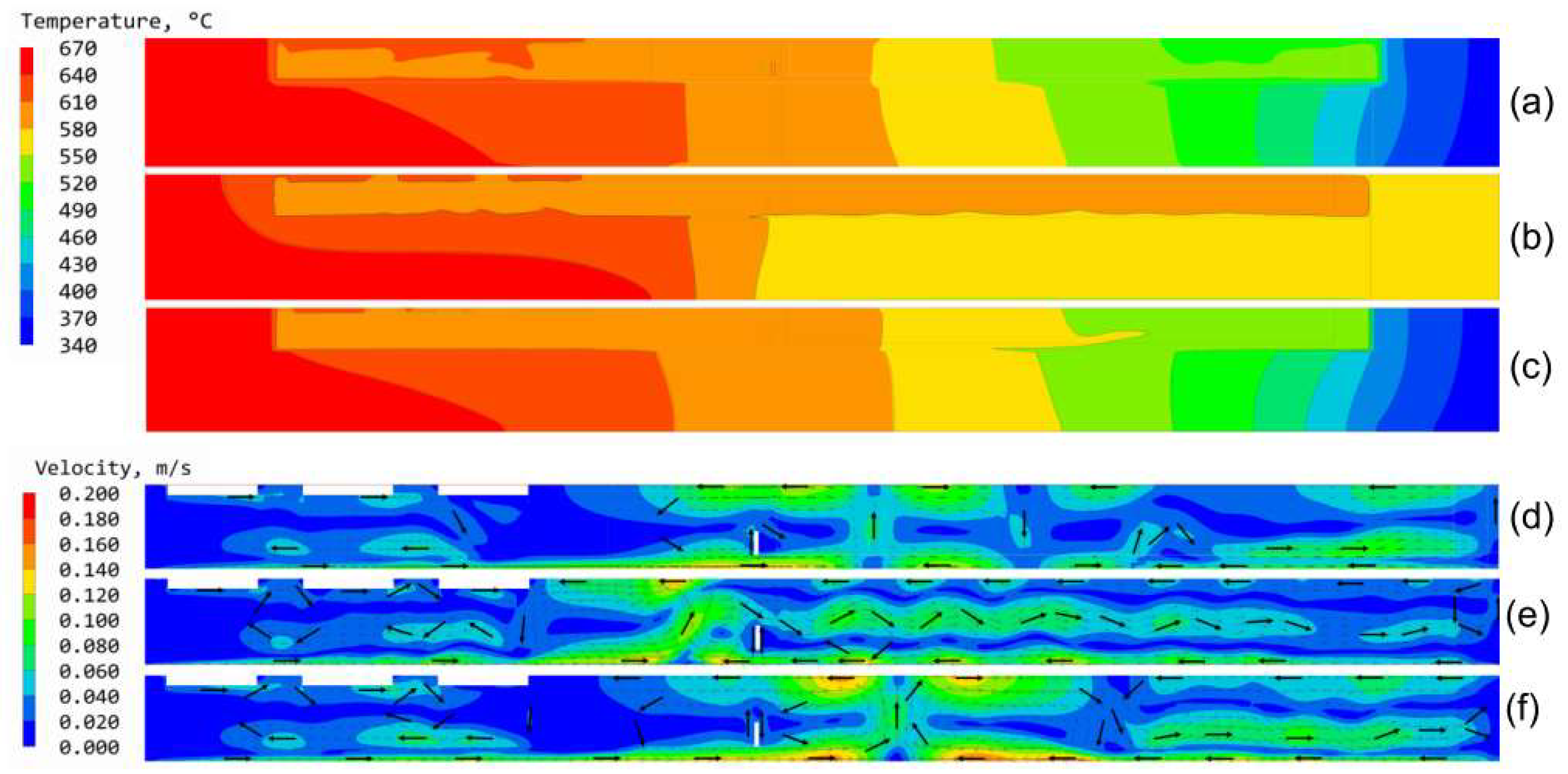
3.6. Results
4. Simulations of the GaN Crystal Growth Process
5. Approaches to Validation
6. Open Questions That May Affect the Accuracy of Simulation Results
6.1. Fluid Properties
6.2. Possible Relevance of Solutal Buoyancy
6.3. Solubility of the Metal
6.4. Dissolution and Growth Kinetics
7. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Juza, R.; Jacobs, H.; Gerke, H. Ammonothermalsynthese von Metallamiden und Metallnitriden. Berichte der Bunsengesellschaft für Phys. Chemie 1966, 70, 1103–1105. [Google Scholar] [CrossRef]
- Dwiliński, R.; Wysmołek, A.; Baranowski, J.; Kamińska, M.; Doradziński, R.; Garczyński, J.; Sierzputowski, L.; Jacobs, H. GaN Synthesis by Ammonothermal Method. Acta Phys. Pol. A 1995, 88, 833–836. [Google Scholar] [CrossRef]
- Pimputkar, S.; Kawabata, S.; Speck, J.S.S.; Nakamura, S. Improved Growth Rates and Purity of Basic Ammonothermal GaN. J. Cryst. Growth 2014, 403, 7–17. [Google Scholar] [CrossRef]
- Bao, Q.; Saito, M.; Hazu, K.; Furusawa, K.; Kagamitani, Y.; Kayano, R.; Tomida, D.; Qiao, K.; Ishiguro, T.; Yokoyama, C.; et al. Ammonothermal Crystal Growth of GaN Using an NH 4 F Mineralizer. Cryst. Growth Des. 2013, 13, 4158–4161. [Google Scholar] [CrossRef]
- Zajac, M.; Kucharski, R.; Grabianska, K.; Gwardys-bak, A.; Puchalski, A.; Wasik, D.; Litwin-Staszewska, E.; Piotrzkowski, R.; Z Domagala, J.; Bockowski, M. Basic Ammonothermal Growth of Gallium Nitride–State of the Art, Challenges, Perspectives. Prog. Cryst. Growth Charact. Mater. 2018, 64, 63–74. [Google Scholar] [CrossRef]
- Key, D.; Letts, E.; Tsou, C.-W.; Ji, M.-H.; Bakhtiary-Noodeh, M.; Detchprohm, T.; Shen, S.-C.; Dupuis, R.; Hashimoto, T. Structural and Electrical Characterization of 2” Ammonothermal Free-Standing GaN Wafers. Progress toward Pilot Production. Materials 2019, 12, 1925. [Google Scholar] [CrossRef] [Green Version]
- Li, T.; Ren, G.; Su, X.; Yao, J.; Yan, Z.; Gao, X.; Xu, K. Growth Behavior of Ammonothermal GaN Crystals Grown on Non-polar and Semi-polar HVPE GaN Seeds. CrystEngComm 2019, 21, 4874–4879. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Pakalapati, R.T.; Kamber, D.S.; Jiang, W.; Pocius, D.W.; Downey, B.C.; McLaurin, M.; D’Evelyn, M.P. High Quality, Low Cost Ammonothermal Bulk GaN Substrates. Jpn. J. Appl. Phys. 2013, 52, 08JA01. [Google Scholar] [CrossRef]
- Wang, B.; Callahan, M.J. Ammonothermal Synthesis of III-nitride Crystals. Cryst. Growth Des. 2006, 6, 1227–1246. [Google Scholar] [CrossRef]
- Dwiliński, R.; Doradziński, R.; Garczyński, J.; Sierzputowski, L.P.; Puchalski, A.; Kanbara, Y.; Yagi, K.; Minakuchi, H.; Hayashi, H. Excellent Crystallinity of Truly Bulk Ammonothermal GaN. J. Cryst. Growth 2008, 310, 3911–3916. [Google Scholar] [CrossRef]
- Suihkonen, S.; Pimputkar, S.; Sintonen, S.; Tuomisto, F. Defects in Single Crystalline Ammonothermal Gallium Nitride. Adv. Electron. Mater. 2017, 3. [Google Scholar] [CrossRef] [Green Version]
- Amano, H. Growth of GaN Layers on Sapphire by Low-Temperature-Deposited Buffer Layers and Realization of p-type GaN by Magesium Doping and Electron Beam Irradiation ( Nobel Lecture ). Angew. Chemie Int. Ed. 2015, 54, 7764–7769. [Google Scholar] [CrossRef] [PubMed]
- Kucharski, R.; Sochacki, T.; Lucznik, B.; Bockowski, M. Growth of Bulk GaN Crystals. J. Appl. Phys. 2020, 128. [Google Scholar] [CrossRef]
- Paskova, T.; Evans, K.R. GaN Substrates-progress, Status, and Prospects. IEEE J. Sel. Top. Quantum Electron. 2009, 15, 1041–1052. [Google Scholar] [CrossRef]
- Amano, H. Progress and Prospect of the Growth of Wide-Band-Gap Group III Nitrides: Development of the Growth Method for Single-Crystal Bulk GaN. Jpn. J. Appl. Phys. 2013, 52, 050001. [Google Scholar] [CrossRef] [Green Version]
- Amano, H.; Baines, Y.; Beam, E.; Borga, M.; Bouchet, T.; Chalker, P.R.; Charles, M.; Chen, K.J.; Chowdhury, N.; Chu, R.; et al. The 2018 GaN Power Electronics Roadmap. J. Phys. D. Appl. Phys. 2018, 51, 163001. [Google Scholar] [CrossRef]
- Fukuda, T.; Ehrentraut, D. Prospects for the Ammonothermal Growth of Large GaN Crystal. J. Cryst. Growth 2007, 305, 304–310. [Google Scholar] [CrossRef]
- Grabianska, K.; Kucharski, R.; Puchalski, A.; Sochacki, T.; Bockowski, M. Recent Progress in Basic Ammonothermal GaN Crystal Growth. J. Cryst. Growth 2020, 547, 125804. [Google Scholar] [CrossRef]
- Tomida, D.; Bao, Q.; Saito, M.; Osanai, R.; Shima, K.; Kojima, K.; Ishiguro, T.; Chichibu, S.F. Ammonothermal Growth of 2 Inch Long GaN Single Crystals Using an Acidic NH4F Mineralizer in a Ag-lined Autoclave. Appl. Phys. Express 2020, 13, 055505. [Google Scholar] [CrossRef]
- Mikawa, Y.; Ishinabe, T.; Kagamitani, Y.; Mochizuki, T.; Ikeda, H.; Iso, K.; Takahashi, T.; Kubota, K.; Enatsu, Y.; Tsukada, Y.; et al. Recent Progress of Large Size and Low Dislocation Bulk GaN Growth. In Proceedings of SPIE, Proceedings of the Gallium Nitride Materials and Devices XV, San Francisco, CA, USA, 4–6 February 2020; Morkoç, H., Fujioka, H., Schwarz, U.T., Eds.; SPIE-International Society for Optics and Photonics: Bellingham, WA, USA; Volume 1128002, p. 1.
- Häusler, J.; Schnick, W. Ammonothermal Synthesis of Nitrides: Recent Developments and Future Perspectives. Chem. A Eur. J. 2018, 24, 11864–11879. [Google Scholar] [CrossRef]
- Richter, T.; Niewa, R. Chemistry of Ammonothermal Synthesis. Inorganics 2014, 2, 29–78. [Google Scholar] [CrossRef] [Green Version]
- Hertrampf, J.; Becker, P.; Widenmeyer, M.; Weidenkaff, A.; Schlücker, E.; Niewa, R. Ammonothermal Crystal Growth of Indium Nitride. Cryst. Growth Des. 2018, 18, 2365–2369. [Google Scholar] [CrossRef]
- Mallmann, M.; Niklaus, R.; Rackl, T.; Benz, M.; Chau, T.G.; Johrendt, D.; Minár, J.; Schnick, W. Solid Solutions of Grimm-Sommerfeld Analogous Nitride Semiconductors II-IV-N2 with II = Mg, Mn, Zn; IV = Si, Ge – Ammonothermal Synthesis and DFT Calculations. Chem. A Eur. J. 2019, 2, 15887–15895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masuda, Y.; Sato, O.; Tomida, D.; Yokoyama, C. Convection Patterns and Temperature Fields of Ammonothermal GaN Bulk Crystal Growth Process. Jpn. J. Appl. Phys. 2016, 55, 3–6. [Google Scholar] [CrossRef]
- Erlekampf, J.; Seebeck, J.; Savva, P.; Meissner, E.; Friedrich, J.; Alt, N.S.A.; Schlücker, E.; Frey, L. Numerical Time-dependent 3D Simulation of Flow Pattern and Heat Distribution in an Ammonothermal System with Various Baffle Shapes. J. Cryst. Growth 2014, 403, 96–104. [Google Scholar] [CrossRef]
- Jiang, Y.N.; Chen, Q.S.; Prasad, V. Numerical Simulation of Ammonothermal Growth processes of GaN Crystals. J. Cryst. Growth 2011, 318, 411–414. [Google Scholar] [CrossRef] [Green Version]
- Moldovan, S.I.; Balasoiu, A.M.; Braun, M. Experimental Investigation of Natural Convection Flow in a Laterally Heated Vertical Cylindrical Enclosure. Int. J. Heat Mass Transf. 2019, 139, 205–212. [Google Scholar] [CrossRef]
- Mirzaee, I.; Charmchi, M.; Sun, H. Heat, Mass, and Crystal Growth of GaN in the Ammonothermal Process: A Numerical Study. Numer. Heat Transf. Part A Appl. 2016, 70, 460–491. [Google Scholar] [CrossRef]
- Pendurti, S.; Chen, Q.S.; Prasad, V. Modeling Ammonothermal Growth of GaN Single Crystals: The Role of Transport. J. Cryst. Growth 2006, 296, 150–158. [Google Scholar] [CrossRef] [Green Version]
- Enayati, H.; Chandy, A.J.; Braun, M.J.; Horning, N. 3D Large Eddy Simulation (LES) Calculations and Experiments of Natural Convection in a Laterally-heated Cylindrical Enclosure for Crystal Growth. Int. J. Therm. Sci. 2017, 116, 1–21. [Google Scholar] [CrossRef]
- Keyes, D.E.; McInnes, L.C.; Woodward, C.; Gropp, W.; Myra, E.; Pernice, M.; Bell, J.; Brown, J.; Clo, A.; Connors, J.; et al. Multiphysics Simulations: Challenges and Opportunities. Int. J. High Perform. Comput. Appl. 2013, 27, 4–83. [Google Scholar] [CrossRef] [Green Version]
- John, B.; Senthilkumar, P.; Sadasivan, S. Applied and Theoretical Aspects of Conjugate Heat Transfer Analysis: A Review. Arch. Comput. Methods Eng. 2019, 26, 475–489. [Google Scholar] [CrossRef]
- Nägel, A.; Logashenko, D.; Schroder, J.B.; Yang, U.M. Aspects of Solvers for Large-Scale Coupled Problems in Porous Media. Transp. Porous Media 2019, 130, 363–390. [Google Scholar] [CrossRef]
- Zhang, S.; Alt, N.S.A.; Schlücker, E.; Niewa, R. Novel Alkali Metal Amidogallates as Intermediates in Ammonothermal GaN Crystal Growth. J. Cryst. Growth 2014, 403, 22–28. [Google Scholar] [CrossRef]
- Zhang, S.; Hintze, F.; Schnick, W.; Niewa, R. Intermediates in Ammonothermal GaN Crystal Growth under Ammonoacidic Conditions. Eur. J. Inorg. Chem. 2013, 5387–5399. [Google Scholar] [CrossRef]
- Hertrampf, J.; Schlücker, E.; Gudat, D.; Niewa, R. Dissolved Intermediates in Ammonothermal Crystal Growth: Stepwise Condensation of [Ga(NH 2) 4]−toward GaN. Cryst. Growth Des. 2017, 17, 4855–4863. [Google Scholar] [CrossRef]
- Becker, P.; Wonglakhon, T.; Zahn, D.; Gudat, D.; Niewa, R. Approaching Dissolved Species in Ammonoacidic GaN Crystal Growth: A Combined Solution NMR and Computational Study. Chem. A Eur. J. 2020, 26, 7008–7017. [Google Scholar] [CrossRef]
- Schimmel, S.; Duchstein, P.; Steigerwald, T.G.; Kimmel, A.-C.L.; Schlücker, E.; Zahn, D.; Niewa, R.; Wellmann, P. In situ X-ray Monitoring of Transport and Chemistry of Ga-containing Intermediates under Ammonothermal Growth Conditions of GaN. J. Cryst. Growth 2018, 498, 214–223. [Google Scholar] [CrossRef]
- Yoshida, K.; Aoki, K.; Fukuda, T. High-temperature Acidic Ammonothermal Method for GaN Crystal Growth. J. Cryst. Growth 2014, 393, 93–97. [Google Scholar] [CrossRef]
- Grabianska, K.; Jaroszynski, P.; Sidor, A.; Bockowski, M.; Iwinska, M. GaN Single Crystalline Substrates by Ammonothermal and HVPE Methods for Electronic Devices. Electronics 2020, 9, 1342. [Google Scholar] [CrossRef]
- Chen, Q.S.; Pendurti, S.; Prasad, V. Modeling of Ammonothermal Growth of Gallium Nitride Single Crystals. J. Mater. Sci. 2006, 41, 1409–1414. [Google Scholar] [CrossRef] [Green Version]
- Schimmel, S.; Kobelt, I.; Heinlein, L.; Kimmel, A.L.; Steigerwald, T.G.; Schlücker, E.; Wellmann, P. Flow Stability, Convective Heat Transfer and Chemical Reactions in Ammonothermal Autoclaves—Insights by In Situ Measurements of Fluid Temperatures. Crystals 2020, 10, 723. [Google Scholar] [CrossRef]
- Griffiths, S.; Pimputkar, S.; Kearns, J.; Malkowski, T.F.; Doherty, M.F.; Speck, J.S.; Nakamura, S. Growth Kinetics of Basic Ammonothermal Gallium Nitride Crystals. J. Cryst. Growth 2018, 501, 74–80. [Google Scholar] [CrossRef]
- Malkowski, T.F.; Pimputkar, S.; Speck, J.S.; DenBaars, S.P.; Nakamura, S. Acidic Ammonothermal Growth of Gallium Nitride in a Liner-free Molybdenum Alloy Autoclave. J. Cryst. Growth 2016, 456, 21–26. [Google Scholar] [CrossRef] [Green Version]
- Schimmel, S. In situ Visualisierung des Ammonothermalen Kristallisationsprozesses Mittels Röntgenmesstechnik. Ph.D. Thesis, Friedrich-Alexander-Universität (FAU), Erlangen-Nürnberg, Germany, 2018. Available online: urn:nbn:de:bvb:29-opus4-102649 (accessed on 29 March 2021).
- Tomida, D.; Kagamitani, Y.; Bao, Q.; Hazu, K.; Sawayama, H.; Chichibu, S.F.; Yokoyama, C.; Fukuda, T.; Ishiguro, T. Enhanced Growth Rate for Ammonothermal Gallium Nitride Crystal Growth Using Ammonium Iodide Mineralizer. J. Cryst. Growth 2012, 353, 59–62. [Google Scholar] [CrossRef]
- Letts, E.; Hashimoto, T.; Hoff, S.; Key, D.; Male, K.; Michaels, M. Development of GaN wafers via the Ammonothermal Method. J. Cryst. Growth 2014, 403, 3–6. [Google Scholar] [CrossRef]
- Enayati, H.; Chandy, A.J.; Braun, M.J. Numerical Simulations of Transitional and Turbulent Natural Convection in Laterally Heated Cylindrical Enclosures for Crystal Growth. Numer. Heat Transf. Part A Appl. 2016, 70, 1195–1212. [Google Scholar] [CrossRef]
- Chen, Q.S.; Pendurti, S.; Prasad, V. Effects of Baffle Design on Fluid Flow and Heat Transfer in Ammonothermal Growth of Nitrides. J. Cryst. Growth 2004, 266, 271–277. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Suzuki, A.; Mikawa, Y.; Kagamitani, Y.; Ishiguro, T.; Yokoyama, C.; Tsukada, T. Numerical Simulation of GaN Single-crystal Growth Process in Ammonothermal Autoclave—Effects of Baffle Shape. Int. J. Heat Mass Transf. 2010, 53, 940–943. [Google Scholar] [CrossRef]
- Chen, Q.-S.; Jiang, Y.-N.; Yan, J.-Y.; Li, W.; Prasad, V. Modeling of Ammonothermal Growth Processes of GaN Crystal in Large-size Pressure Systems. Res. Chem. Intermed. 2011, 37, 467–477. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Suzuki, A.; Ishiguro, T.; Yokoyama, C. Numerical Simulation of Heat and Fluid Flow in Ammonothermal GaN Bulk Crystal Growth Process. Jpn. J. Appl. Phys. 2013, 52, 08JA05. [Google Scholar] [CrossRef]
- Chen, Q.-S.; Prasad, V.; Hu, W.R. Modeling of Ammonothermal Growth of Nitrides. J. Cryst. Growth 2003, 258, 181–187. [Google Scholar] [CrossRef]
- Moldovan, S.I. Numerical Simulation and Experimental Validation of Fluid Flow and Mass Transfer in an Ammonothermal Crystal Growth Reactor. Ph.D. Thesis, University of Akron, Akron, OH, USA, May 2013. [Google Scholar]
- Schimmel, S.; Koch, M.; Macher, P.; Kimmel, A.C.L.; Steigerwald, T.G.; Alt, N.S.A.; Schlücker, E.; Wellmann, P. Solubility and Dissolution Kinetics of GaN in Supercritical Ammonia in Presence of Ammonoacidic and Ammonobasic Mineralizers. J. Cryst. Growth 2017, 479, 59–66. [Google Scholar] [CrossRef]
- Schimmel, S.; Tomida, D.; Saito, M.; Bao, Q.; Ishiguro, T.; Honda, Y.; Chichibu, S.; Amano, H. Boundary Conditions for Simulations of Fluid Flow and Temperature Field during Ammonothermal Crystal Growth—A Machine-Learning Assisted Study of Autoclave Wall Temperature Distribution. Crystals 2021, 11, 254. [Google Scholar] [CrossRef]
- Chen, Q.; Jiang, Y.; Yan, J.; Qin, M. Progress in modeling of Fluid Flows in Crystal Growth Processes. Prog. Nat. Sci. 2008, 18, 1465–1473. [Google Scholar] [CrossRef]
- Li, H.; Braun, M.J.; Xing, C. Fluid Flow and Heat Transfer in a Cylindrical Model Hydrothermal Reactor. J. Cryst. Growth 2006, 289, 207–216. [Google Scholar] [CrossRef]
- Enayati, H. Effect of Reactor Size in a Laterally-Heated Cylindrical Reactor. Int. J. Heat Technol. 2020, 38, 275–284. [Google Scholar] [CrossRef]
- Li, H.; Evans, E.A.; Wang, G.X. Flow of Solution in Hydrothermal Autoclaves with Various Aspect Ratios. J. Cryst. Growth 2003, 256, 146–155. [Google Scholar] [CrossRef]
- Ursu, D.; Negrila, R.; Popescu, A.; Grozescu, I.; Vizman, D. Numerical and Experimental Studies of Fluid Flow and Heat Transfer in a Model Experiment for Hydrothermal Growth. Solid State Phenom. 2016, 254, 237–242. [Google Scholar] [CrossRef]
- Hirsch, C. Numerical Computation of Internal and External Flows, 2nd ed.; Elsevier: Burlington, MA, USA, 2007; ISBN 9780750665940. [Google Scholar]
- Alfonsi, G. Reynolds-averaged Navier-Stokes Equations for Turbulence Modeling. Appl. Mech. Rev. 2009, 62, 1–20. [Google Scholar] [CrossRef]
- Enayati, H.; Chandy, A.; Braun, M.J. Three-dimensional Large Eddy Simulations of Natural Convection in Laterally Heated Cylindrical Enclosures with Racks and Seeds for Crystal Growth. In Proceedings of the Second Thermal and Fluids Engineering Conference, Las Vegas, NV, USA, 2–5 April 2017; pp. 719–732. [Google Scholar]
- Masuda, Y.; Suzuki, A.; Mikawa, Y.; Chani, V.; Yokoyama, C.; Tsukada, T. Numerical Simulation of Hydrothermal Autoclave for Single-Crystal Growth Process. J. Therm. Sci. Technol. 2008, 3, 540–551. [Google Scholar] [CrossRef] [Green Version]
- Enayati, H.; Braun, M.J.; Chandy, A.J. Numerical Simulations of Porous Medium with Different Permeabilities and Positions in a Laterally-heated Cylindrical Enclosure for Crystal Growth. J. Cryst. Growth 2018, 483, 65–80. [Google Scholar] [CrossRef]
- Malin, M.R. Brian Spalding: Some Contributions to Computational Fluid Dynamics During the Period 1993 to 2004. In 50 Years of CFD in Engineering Sciences; Springer: Singapore, 2020; pp. 3–39. [Google Scholar]
- Xiao, H.; Cinnella, P. Quantification of Model Uncertainty in RANS Simulations: A Review. Prog. Aerosp. Sci. 2019, 108, 1–31. [Google Scholar] [CrossRef] [Green Version]
- Pimputkar, S.; Speck, J.S.; Nakamura, S. Basic Ammonothermal GaN Growth in Molybdenum Capsules. J. Cryst. Growth 2016, 456, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Springer Handbook of Crystal Growth; Dhanaraj, G.; Byrappa, K.; Prasad, V.; Dudley, M. (Eds.) Springer: Berlin/Heidelberg, Germany, 2010; ISBN 978-3-540-74182-4. [Google Scholar]
- Prasad, V.; Kulacki, F.A.; Keyhani, M. Natural Convection in Porous Media. J. Fluid Mech. 1985, 150, 89–119. [Google Scholar] [CrossRef]
- Alloui, Z.; Vasseur, P. Convection in Superposed Fluid and Porous Layers. Acta Mech. 2010, 214, 245–260. [Google Scholar] [CrossRef]
- Battiato, I.; Ferrero V, P.T.; O’ Malley, D.; Miller, C.T.; Takhar, P.S.; Valdés-Parada, F.J.; Wood, B.D. Theory and Applications of Macroscale Models in Porous Media. Transp. Porous Media 2019, 130, 5–76. [Google Scholar] [CrossRef]
- Mao, D.; Karanikas, J.M.; Fair, P.S.; Prodan, I.D.; Wong, G.K. A Different Perspective on the Forchheimer and Ergun Equations. SPE J. 2016, 21, 1501–1507. [Google Scholar] [CrossRef]
- Papathanasiou, T.D.; Markicevic, B.; Dendy, E.D. A Computational Evaluation of the Ergun and Forchheimer Equations for Fibrous Porous Media. Phys. Fluids 2001, 13, 2795–2804. [Google Scholar] [CrossRef]
- Auriault, J.-L. On the Domain of Validity of Brinkman’s Equation. Transp. Porous Media 2009, 79, 215–223. [Google Scholar] [CrossRef]
- McBride, D.; Croft, T.N.; Cross, M. A Coupled Finite Volume Method for the Computational Modelling of Mould Filling in Very Complex Geometries. Comput. Fluids 2008, 37, 170–180. [Google Scholar] [CrossRef]
- Gohil, T.; McGregor, R.H.P.; Szczerba, D.; Burckhardt, K.; Muralidhar, K.; Székely, G. Simulation of Oscillatory Flow in an Aortic Bifurcation Using FVM and FEM: A Comparative Study of Implementation Strategies. Int. J. Numer. Methods Fluids 2011, 66, 1037–1067. [Google Scholar] [CrossRef]
- Jeong, W.; Seong, J. Comparison of Effects on Technical Variances of Computational Fluid Dynamics (CFD) Software Based on Finite Element and Finite Volume Methods. Int. J. Mech. Sci. 2014, 78, 19–26. [Google Scholar] [CrossRef]
- Molina-Aiz, F.D.; Fatnassi, H.; Boulard, T.; Roy, J.C.; Valera, D.L. Comparison of Finite Element and Finite Volume Methods for Simulation of Natural Ventilation in Greenhouses. Comput. Electron. Agric. 2010, 72, 69–86. [Google Scholar] [CrossRef]
- Bern, M.; Plassmann, P. Mesh Generation. In Handbook of Computational Geometry; North Holland: Amsterdam, The Netherlands, 2000. [Google Scholar]
- Mirzaee, I. Computational Investigation of Gallium Nitrite Ammonothermal Crystal Growth. Ph.D. Thesis, University of Massachusetts Lowell, Lowell, MA, USA, 2015. [Google Scholar]
- Li, H.; Evans, E.A.; Wang, G.X. A Three-dimensional Conjugate Model with Realistic Boundary Conditions for Flow and Heat Transfer in an Industry Scale Hydrothermal Autoclave. Int. J. Heat Mass Transf. 2005, 48, 5166–5178. [Google Scholar] [CrossRef]
- Zajac, M.; Kucharski, R.; Grabiańska, K.; Gwardys-Bąk, A.; Puchalski, A.; Boćkowski, M. Ammonothermal GaN Substrates for Microwave Electronics and Energoelectronics. In Proceedings of SPIE, Proceedings of the Radioelectronics Systems Conference, Jachranka, Poland, 14–16 November 2017; SPIE-International Society for Optics and Photonics: Bellingham, WA, USA. [CrossRef]
- Pimputkar, S.; Kawabata, S.; Speck, J.S.; Nakamura, S. Surface Morphology Study of Basic Ammonothermal GaN Grown on Non-polar GaN Seed Crystals of Varying Surface Orientations from m-plane to a-plane. J. Cryst. Growth 2013, 368, 67–71. [Google Scholar] [CrossRef]
- Alt, N.; Meissner, E.; Schlücker, E.; Frey, L. In situ Monitoring Technologies for Ammonthermal Reactors. Phys. Status Solidi Curr. Top. Solid State Phys. 2012, 9, 436–439. [Google Scholar] [CrossRef]
- Steigerwald, T.G.; Balouschek, J.; Hertweck, B.; Kimmel, A.-C.L.; Alt, N.S.A.; Schluecker, E. In situ Investigation of Decomposing Ammonia and Ammonobasic Solutions under Supercritical Conditions via UV/vis and Raman Spectroscopy. J. Supercrit. Fluids 2018, 134, 96–105. [Google Scholar] [CrossRef]
- Schimmel, S.; Lindner, M.; Steigerwald, T.G.; Hertweck, B.; Richter, T.M.M.; Künecke, U.; Alt, N.S.A.; Niewa, R.; Schlücker, E.; Wellmann, P.J. Determination of GaN Solubility in Supercritical Ammonia with NH4F and NH4Cl Mineralizer by in situ X-ray Imaging of Crystal Dissolution. J. Cryst. Growth 2015, 418, 64–69. [Google Scholar] [CrossRef]
- Schimmel, S.; Künecke, U.; Meisel, M.; Hertweck, B.; Steigerwald, T.G.; Nebel, C.; Alt, N.S.A.; Schlücker, E.; Wellmann, P. Chemical Stability of Carbon-based Inorganic Materials for in situ X-ray Investigations of Ammonothermal Crystal Growth of Nitrides. J. Cryst. Growth 2016, 456, 33–42. [Google Scholar] [CrossRef]
- Schimmel, S.; Wellmann, P. In situ Visualization of the Ammonothermal Crystallization Process by X-ray Technology. In Ammonothermal Synthesis and Crystal Growth of Nitrides–Chemistry and Technology; Niewa, R., Meissner, E., Eds.; Springer International Publishing: Berlin, Germany, 2021; Volume 304, ISBN 978-3-030-56305-9. [Google Scholar]
- Beckermann, C.; Viskanta, R.; Ramadhyani, S. Natural Convection in Vertical Enclosures Containing Simultaneously Fluid and Porous Layers. J. Fluid Mech. 1988, 186, 257–284. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Callahan, M.J.; Rakes, K.D.; Bouthillette, L.O.; Wang, S.Q.; Bliss, D.F.; Kolis, J.W. Ammonothermal Growth of GaN Crystals in Alkaline Solutions. J. Cryst. Growth 2006, 287, 376–380. [Google Scholar] [CrossRef]
- Griffiths, S.; Pimputkar, S.; Speck, J.S.; Nakamura, S. On the Solubility of Gallium Nitride in Supercritical Ammonia–sodium Solutions. J. Cryst. Growth 2016, 456, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Mirzaii, I.; Passandideh-Fard, M. Modeling Free Surface Flows in Presence of an Arbitrary Moving Object. Int. J. Multiph. Flow 2012, 39, 216–226. [Google Scholar] [CrossRef]
- Liu, X.; Harada, H.; Miyamura, Y.; Han, X.F.; Nakano, S.; Nishizawa, S.I.; Kakimoto, K. Transient Global Modeling for the Pulling Process of Czochralski Silicon Crystal Growth. I. Principles, Formulation, and Implementation of the Model. J. Cryst. Growth 2020, 532, 125405. [Google Scholar] [CrossRef]
- Dadzis, K.; Pätzold, O.; Gerbeth, G. Model Experiments for Flow Phenomena in Crystal Growth. Cryst. Res. Technol. 2020, 55, 1–7. [Google Scholar] [CrossRef]
- Lemmon, E.W.; McLinden, M.O.; Friend, D.G. Thermophysical Properties of Fluid Systems; Linstrom, P.J., Mallard, W.G., Eds.; Available online: http://http//webbook.nist.gov/chemistry (accessed on 5 June 2017).
- Tomida, D.; Yoshinaga, T. Thermal Conductivity Measurements of Liquid Ammonia by the Transient Short-Hot-Wire Method. Int. J. Thermophys. 2020, 41, 53. [Google Scholar] [CrossRef]
- Brennecke, J.F.; Eckert, C.A. Phase Equilibria for Supercritical Fluid Process Design. AIChE J. 1989, 35, 1409–1427. [Google Scholar] [CrossRef]
- Supercritical Fluids; Arai, Y.; Sako, T.; Takebayashi, Y. (Eds.) Springer: Berlin/Heidelberg, Germany, 2002; ISBN 978-3-642-62515-2. [Google Scholar]
- Pimputkar, S.; Nakamura, S. Decomposition of Supercritical Ammonia and Modeling of Supercritical Ammonia-nitrogen-hydrogen Solutions with Applicability toward Ammonothermal Conditions. J. Supercrit. Fluids 2016, 107, 17–30. [Google Scholar] [CrossRef] [Green Version]
- Hosaka, M.; Taki, S. Hydrothermal Growth of Quartz Crystals in Pure Water. J. Cryst. Growth 1981, 51, 640–642. [Google Scholar] [CrossRef]
- Hervey, P.R.; Foise, J.W. Syntheic Quartz Crystal—A Review. Miner. Metall. Process. 2001, 18, 1–4. [Google Scholar]
- Ehrentraut, D.; Sato, H.; Kagamitani, Y.; Sato, H.; Yoshikawa, A.; Fukuda, T. Solvothermal Growth of ZnO. Prog. Cryst. Growth Charact. Mater. 2006, 52, 280–335. [Google Scholar] [CrossRef]
- Bao, Q.; Saito, M.; Hazu, K.; Kagamitani, Y.; Kurimoto, K.; Tomida, D.; Qiao, K.; Ishiguro, T.; Yokoyama, C.; Chichibu, S.F. Ammonothermal Growth of GaN on a Self-nucleated GaN Seed Crystal. J. Cryst. Growth 2014, 404, 168–171. [Google Scholar] [CrossRef]
- Duan, X.; Qian, G.; Fan, C.; Zhu, Y.; Zhou, X.; Chen, D.; Yuan, W. First-principles Calculations of Ammonia Decomposition on Ni(110) Surface. Surf. Sci. 2012, 606, 549–553. [Google Scholar] [CrossRef]
- D’Evelyn, M.P.; Ehrentraut, D.; Jiang, W.; Kamber, D.S.; Downey, B.C.; Pakalapati, R.T.; Yoo, H.D. Ammonothermal Bulk GaN Substrates for Power Electronics. ECS Trans. 2013, 58, 287–294. [Google Scholar] [CrossRef]
- Hertweck, B.; Schimmel, S.; Steigerwald, T.G.; Alt, N.S.A.; Wellmann, P.J.; Schluecker, E. Ceramic Liner Technology for Ammonoacidic Synthesis. J. Supercrit. Fluids 2015, 99, 76–87. [Google Scholar] [CrossRef]
- Bajus, S. Ammoniakzersetzung mit Salzmodifizierten Katalysatoren. Ph.D. Thesis, Friedrich-Alexander-Universität Erlangen-Nürnberg (FAU), Erlangen-Nürnberg, Germany, June 2014. [Google Scholar]
- Lu, K.; Tatarchuk, B.J. Activated Chemisorption of Hydrogen on Supported Ruthenium. II. Effects of Crystallite Size and Adsorbed Chlorine on Accurate Surface Area Measurements. J. Catal. 1987, 106, 176–187. [Google Scholar] [CrossRef]
- Shlflett, W.K.; Dumeslc, J.A. Ammonia Synthesis as a Catalytic Probe of Supported Ruthenium Catalysts: The Role of the Support and the Effect of Chlorine. Ind. Eng. Chem. Fundam. 1981, 20, 246–250. [Google Scholar] [CrossRef]
- Steigerwald, T.G.; Alt, N.S.A.; Hertweck, B.; Schluecker, E. Feasibility of Density and Viscosity Measurements under Ammonothermal Conditions. J. Cryst. Growth 2014, 403, 59–65. [Google Scholar] [CrossRef]
- Keblinski, P.; Eastman, J.A.; Cahill, D.G. Nanofluids for Thermal Transport. Mater. Today 2005, 8, 36–44. [Google Scholar] [CrossRef]
- Buongiorno, J. Convective Transport in Nanofluids. J. Heat Transfer 2006, 128, 240–250. [Google Scholar] [CrossRef]
- Bänsch, E. A Thermodynamically Consistent Model for Convective Transport in Nanofluids: Existence of Weak Solutions and Fem Computations. J. Math. Anal. Appl. 2019, 477, 41–59. [Google Scholar] [CrossRef]
- Tomida, D.; Kuroda, K.; Nakamura, K.; Qiao, K.; Yokoyama, C. Temperature Dependent Control of the Solubility of Gallium Nitride in Supercritical Ammonia Using Mixed Mineralizer. Chem. Cent. J. 2018, 12, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Tomida, D.; Kuroda, K.; Hoshino, N.; Suzuki, K.; Kagamitani, Y.; Ishiguro, T.; Fukuda, T.; Yokoyama, C. Solubility of GaN in Supercritical Ammonia with Ammonium Chloride as a Mineralizer. J. Cryst. Growth 2010, 312, 3161–3164. [Google Scholar] [CrossRef]
- Bushmin, S.A.; Azimov, P.; Lvov, S. Numerical Modelling of the Metamorphic Mineral Solubility in Hydrothermal Solutions at 400-800 C, 1-5 kbar and Various Fluid Acidity. Mineral. Collect. 2004, 54, 94–116. [Google Scholar]
- Zahn, D. On the Solvation of Metal Ions in Liquid Ammonia: A Molecular Simulation Study of M(NH2)x(NH3)y Complexes as a Function of pH. RSC Adv. 2017, 7, 54063–54067. [Google Scholar] [CrossRef] [Green Version]
- Zahn, D. A Molecular Simulation Study of the Auto-protolysis of Ammonia as a Function of Temperature. Chem. Phys. Lett. 2017, 682, 55–59. [Google Scholar] [CrossRef]
- Ehrentraut, D.; Kagamitani, Y.; Yokoyama, C.; Fukuda, T. Physico-chemical Features of the Acid Ammonothermal Growth of GaN. J. Cryst. Growth 2008, 310, 891–895. [Google Scholar] [CrossRef]
- Hashimoto, T.; Saito, M.; Fujito, K.; Wu, F.; Speck, J.S.; Nakamura, S. Seeded Growth of GaN by the Basic Ammonothermal Method. J. Cryst. Growth 2007, 305, 311–316. [Google Scholar] [CrossRef]
- Hashimoto, T.; Letts, E. Development of Cost-Effective Native Substrates for Gallium Nitride-Based Optoelectronic Devices via Ammonothermal Growth. Optoelectron. Devices Appl. 2012, 95–106. [Google Scholar] [CrossRef] [Green Version]
- Schimmel, S.; Künecke, U.; Baser, H.; Steigerwald, T.G.; Hertweck, B.; Alt, N.S.A.; Schlücker, E.; Schwieger, W.; Wellmann, P. Towards X-ray in-situ Visualization of Ammonothermal Crystal Growth of Nitrides. Phys. Status Solidi Curr. Top. Solid State Phys. 2014, 11, 1439–1442. [Google Scholar] [CrossRef]
- Font, B.; Weymouth, G.D.; Nguyen, V.-T.; Tutty, O.R. Deep Learning of the Spanwise-averaged Navier–Stokes Equations. J. Comput. Phys. 2021, 434, 110199. [Google Scholar] [CrossRef]
- Tsunooka, Y.; Kokubo, N.; Hatasa, G.; Harada, S.; Tagawa, M.; Ujihara, T. High-speed Prediction of Computational Fluid Dynamics Simulation in Crystal Growth. CrystEngComm 2018, 20, 6546–6550. [Google Scholar] [CrossRef] [Green Version]
- Raissi, M.; Perdikaris, P.; Karniadakis, G.E. Physics-informed Neural Networks: A Deep Learning Framework for Solving Forward and Inverse Problems Involving Nonlinear Partial Differential Equations. J. Comput. Phys. 2019, 378, 686–707. [Google Scholar] [CrossRef]
- Givi, P.; Daley, A.J.; Mavriplis, D.; Malik, M. Quantum Speedup for Aeroscience and Engineering. AIAA J. 2020, 58, 3715–3727. [Google Scholar] [CrossRef]
- Bharadwaj, S.S.; Sreenivasan, K.R. Quantum Computation of Fluid Dynamics. 2020. Available online: https://arxiv.org/abs/2007.09147 (accessed on 29 March 2021).
- Bockowski, M.; Iwinska, M.; Amilusik, M.; Fijalkowski, M.; Lucznik, B.; Sochacki, T. Challenges and Future Perspectives in HVPE-GaN Growth on Ammonothermal GaN Seeds. Semicond. Sci. Technol. 2016, 31, 093002. [Google Scholar] [CrossRef]
- Gao, B.; Kakimoto, K. Three-dimensional Modeling of Basal Plane Dislocations in 4H-SiC Single Crystals Grown by the Physical Vapor Transport Method. Cryst. Growth Des. 2014, 14, 1272–1278. [Google Scholar] [CrossRef]



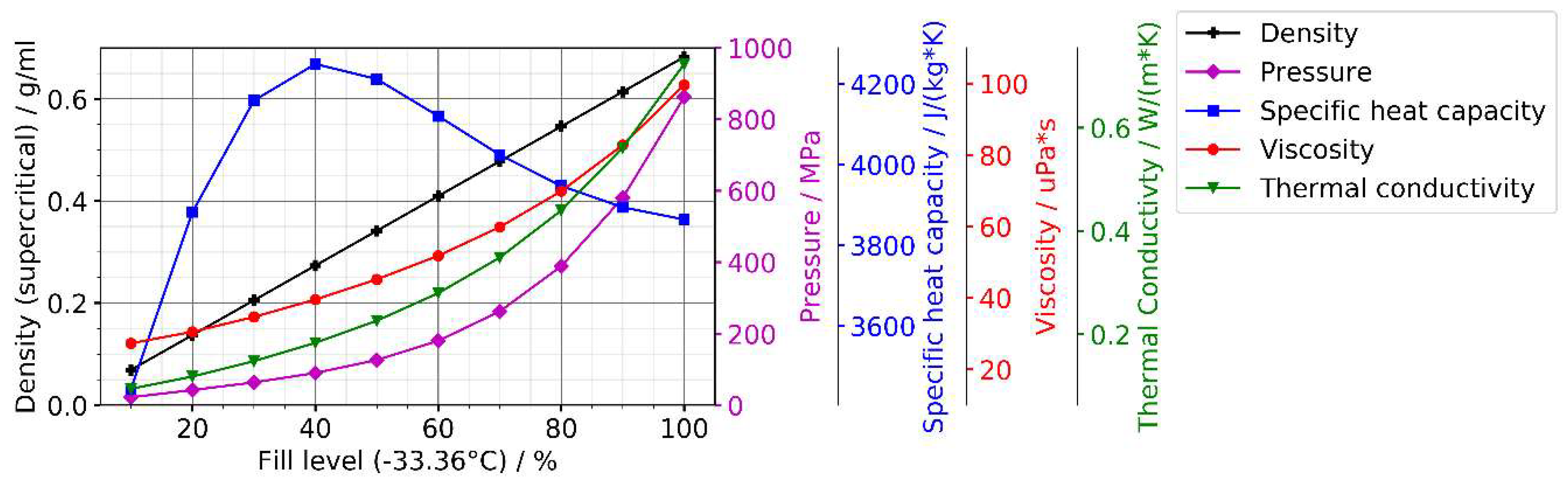


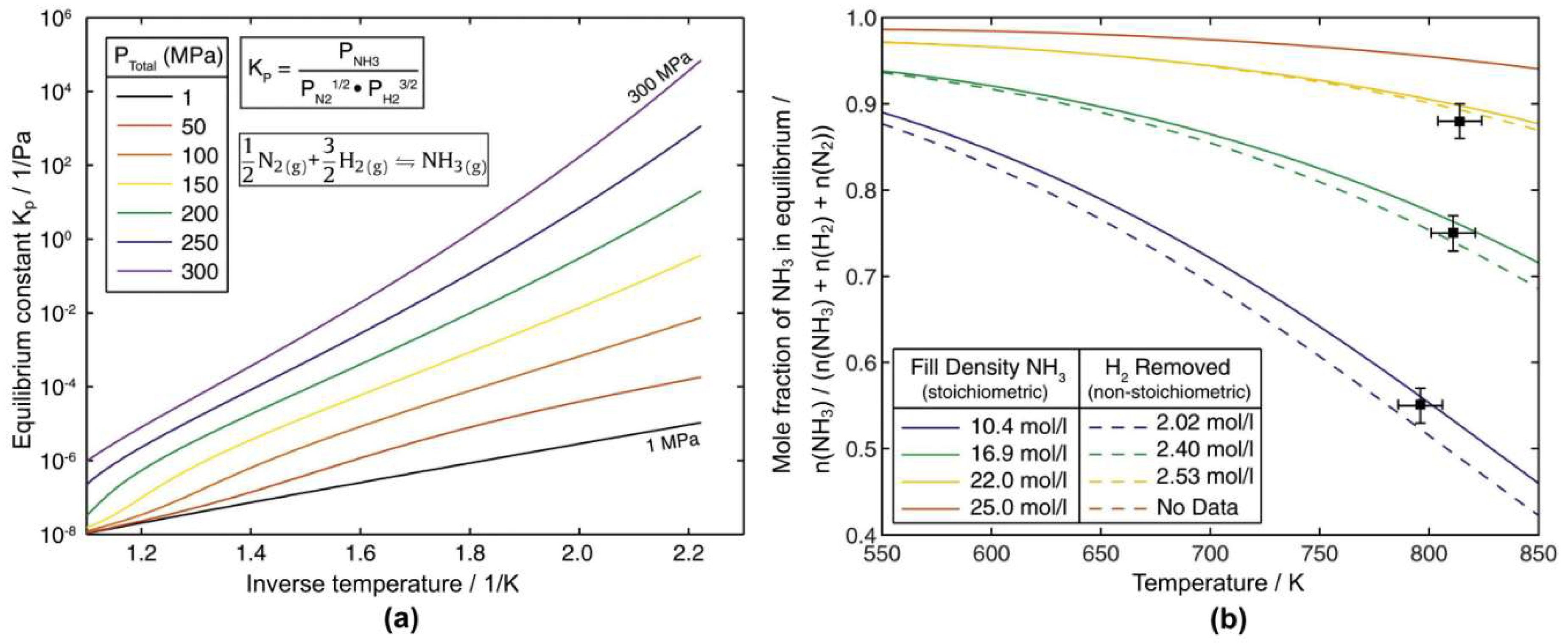
| Hydrothermal | Ammonothermal | |||||
|---|---|---|---|---|---|---|
| Material | Quartz | ZnO | GaN | |||
| Process Route | Mineralizer-Free [103] | Low-Pressure Process [104] | High-Pressure Process [104] | [105] | Acidic [106] | Basic [3] |
| TCZ/°C | 445–500 | 345 | 360 | 300–430 | 625 | 575 |
| TCZ/Tc | 1.19–1.34 | 0.92 | 0.96 | 0.80–1.15 | 4.73 | 4.35 |
| ΔT/°C | 25 | 10 | 25 | 10–20 | 50 | 30–45 |
| Solubility | retrograde | normal | normal | retrograde | ||
| p/MPa | 60–110 1 | 70–100 | 100–150 | 70–255 | 80–150 | 250 |
| p/pc | 2.71–4.98 | 3.17–4.52 | 4.52–6.79 | 3.17–11.54 | 7.08–13.27 | 22.12 |
| Mineralizer | none | Na2CO3 | NaOH | NaOH | NH4F | Na |
| [0001] growth rate/µm/day | 0.3–2 | 400 | 1000 | 300 | 410 | 344 |
| Supercritical NH3 | Dissolved Ga | |
|---|---|---|
| Density difference/mol/L | 1.2–5.8 [44] | 0.5 [39] |
| Density difference/g/L | 20.4–104.6 | 34.9 |
| Mineralizer | Experimental Conditions | Range of Solubility Data | Reference |
|---|---|---|---|
| NH4Cl/NH4I mixture | 450–550 °C (external) 96–102 MPa 0.42–0.51 mmol NH4X/mL (*) 100 h | 0.048–0.052 mol GaN/mol NH4X (*) 0.15–1.2 mol% 0.42–0.47 mmol/mL (*) | D. Tomida [117] |
| NH4Cl/NH4Br mixture | 450–550 °C (external) 96–103 MPa 0.40–0.51 mmol NH4X/mL (*) 100 h | 0.11–0.12 mol GaN/mol NH4X (*) 0.35–1.23 mol% 0.40–0.51 mmol/mL (*) | D. Tomida 2018 [117] |
| NH4Cl | 200–550 °C (external) 67.7–100.9 MPa 0.33–3.30 mmol NH4Cl/mL (*) 120 h | 0–2.4 mol GaN/mol NH4Cl 0–7.04 mol% (*) 0–7.92 mmol/mL (*) | D. Ehrentraut 2008 [122] |
| 420–600 °C (external) 55–150 MPa 0–4.04 mmol NH4Cl/mL (*) 100 h | up to 0.41 mol GaN/mol NH4Cl (*) 0.04–5.47 mol% 0.01–1.65 mmol/mL (*) | D. Tomida 2010 [118] | |
| NH4F | 486–572 °C (internal) 16–175 MPa 0.76 mmol NH4F/mL Until observation of saturation | 0–0.11 mol GaN/mol NH4F 0–1.03 mol% 0–0.08 mmol/mL | S. Schimmel 2017/2018 [56]/[46] |
| Na | 415–650 °C (internal) 200 MPa 14.13–21.89 mmol Na/mL (*) 45–316 h | 0.00017–0.00122 mol GaN/mol Na (*) 0.02–0.12 mol% 0.07–3.45 mmol/mL (*) | S. Griffiths 2016 [94] |
| NaNH2 | 450–650 °C (external) 76 ± 12 MPa 0.14 mmol NaNH2/mL (*) 120 h | up to 0.16 mol GaN/mol NaNH2 (*) up to 2.44 mol% (*) up to 0.02 mmol/mL (*) | T. Hashimoto 2007/2011 [123,124] |
| NaN3 | 396–538 °C (internal) 259–268 MPa 0.65 ± 0.07 mmol NaN3/mL Until observation of saturation | 0.02–0.05 mol GaN/mol NaN3 0.04–0.15 mol% 0.01–0.04 mmol/mL | S. Schimmel 2017/2018 [56]/[46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schimmel, S.; Tomida, D.; Ishiguro, T.; Honda, Y.; Chichibu, S.; Amano, H. Numerical Simulation of Ammonothermal Crystal Growth of GaN—Current State, Challenges, and Prospects. Crystals 2021, 11, 356. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040356
Schimmel S, Tomida D, Ishiguro T, Honda Y, Chichibu S, Amano H. Numerical Simulation of Ammonothermal Crystal Growth of GaN—Current State, Challenges, and Prospects. Crystals. 2021; 11(4):356. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040356
Chicago/Turabian StyleSchimmel, Saskia, Daisuke Tomida, Tohru Ishiguro, Yoshio Honda, Shigefusa Chichibu, and Hiroshi Amano. 2021. "Numerical Simulation of Ammonothermal Crystal Growth of GaN—Current State, Challenges, and Prospects" Crystals 11, no. 4: 356. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040356







