Biochar-Supported BiOx for Effective Electrosynthesis of Formic Acid from Carbon Dioxide Reduction
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis
2.3. Physical and Chemical Characterizations
2.4. Preparation of the Electrodes
2.5. Electrochemical Characterizations for the CO2RR and Product Analysis
3. Results and Discussion
3.1. Physical and Chemical Characterizations of the Synthesized Catalysts
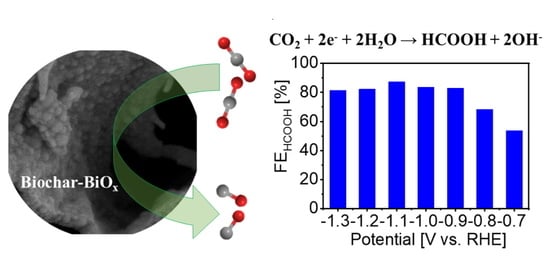
3.2. Electrochemical Properties of the Biochar–BiOx Electrode toward the CO2RR
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Guzmán, H.; Salomone, F.; Batuecas, E.; Tommasi, T.; Russo, N.; Bensaid, S.; Hernández, S. How to make sustainable CO2 conversion to Methanol: Thermocatalytic versus electrocatalytic technology. Chem. Eng. J. 2020, in press. [Google Scholar] [CrossRef]
- François, J.M.; Lachaux, C.; Morin, N. Synthetic Biology Applied to Carbon Conservative and Carbon Dioxide Recycling Pathways. Front. Bioeng. Biotechnol. 2020, 7. [Google Scholar] [CrossRef]
- Bushuyev, O.S.; De Luna, P.; Dinh, C.T.; Tao, L.; Saur, G.; van de Lagemaat, J.; Kelley, S.O.; Sargent, E.H. What Should We Make with CO2 and How Can We Make It? Joule 2018, 2, 825–832. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Rino, T.; Bejtka, K.; Castellino, M.; Sacco, A.; Farkhondehfal, M.A.; Chiodoni, A.; Drago, F.; Pirri, C.F. Coupled Copper–Zinc Catalysts for Electrochemical Reduction of Carbon Dioxide. ChemSusChem 2020, 13, 4128–4139. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef] [PubMed]
- Zeng, J.; Bejtka, K.; Di Martino, G.; Sacco, A.; Castellino, M.; Fiorentin, M.R.; Risplendi, F.; Farkhondehfal, M.A.; Hernández, S.; Cicero, G.; et al. Microwave-Assisted Synthesis of Copper-Based Electrocatalysts for Converting Carbon Dioxide to Tunable Syngas. ChemElectroChem 2020, 7, 229–238. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Smith, W.A.; Burdyny, T. Introductory Guide to Assembling and Operating Gas Diffusion Electrodes for Electrochemical CO2 Reduction. ACS Energy Lett. 2019, 4, 639–643. [Google Scholar] [CrossRef] [Green Version]
- Burdyny, T.; Smith, W.A. CO2 reduction on gas-diffusion electrodes and why catalytic performance must be assessed at commercially-relevant conditions. Energy Environ. Sci. 2019, 12, 1442–1453. [Google Scholar] [CrossRef] [Green Version]
- Zeng, J.; Francia, C.; Dumitrescu, M.A.; Videla, A.H.A.M.; Ijeri, V.S.; Specchia, S.; Spinelli, P. Electrochemical Performance of Pt-Based Catalysts Supported on Different Ordered Mesoporous Carbons (Pt/OMCs) for Oxygen Reduction Reaction. Ind. Eng. Chem. Res. 2012, 51, 7500–7509. [Google Scholar] [CrossRef]
- Guzmán, H.; Farkhondehfal, M.A.; Tolod, K.R.; Hernández, S.; Russo, N. Photo/electrocatalytic hydrogen exploitation for CO2 reduction toward solar fuels production. Solar Hydrog. Prod. 2019, 365–418. [Google Scholar] [CrossRef]
- Zeng, J.; Bejtka, K.; Ju, W.; Castellino, M.; Chiodoni, A.; Sacco, A.; Farkhondehfal, M.A.; Hernández, S.; Rentsch, D.; Battaglia, C.; et al. Advanced Cu-Sn foam for selectively converting CO2 to CO in aqueous solution. Appl. Catal. B: Environ. 2018, 236, 475–482. [Google Scholar] [CrossRef]
- Zhao, G.-X.; Huang, X.; Wang, X.; Wang, X.-K. Progress in catalyst exploration for heterogeneous CO2 reduction and utilization: A critical review. J. Mater. Chem. A 2017, 5, 21625–21649. [Google Scholar] [CrossRef]
- Xie, H.; Wang, T.; Liang, J.; Li, Q.; Sun, S. Cu-based nanocatalysts for electrochemical reduction of CO2. Nano Today 2018, 21, 41–54. [Google Scholar] [CrossRef]
- Yu, X.; Pickup, P.G. Recent advances in direct formic acid fuel cells (DFAFC). J. Power Sources 2008, 182, 124–132. [Google Scholar] [CrossRef]
- Eppinger, J.; Huang, K.-W. Formic Acid as a Hydrogen Energy Carrier. ACS Energy Lett. 2017, 2, 188–195. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Kim, B.; Jhong, H.-R.M.; Ma, S.; Kenis, P.J.A. A Gross-Margin Model for Defining Technoeconomic Benchmarks in the Electroreduction of CO2. ChemSusChem 2016, 9, 1972–1979. [Google Scholar] [CrossRef]
- Hori, Y.; Wakebe, H.; Tsukamoto, T.; Koga, O. Electrocatalytic process of CO selectivity in electrochemical reduction of CO2 at metal electrodes in aqueous media. Electrochimica Acta 1994, 39, 1833–1839. [Google Scholar] [CrossRef]
- Zhao, S.; Li, S.; Guo, T.; Zhang, S.; Wang, J.; Wu, Y.; Chen, Y. Advances in Sn-Based Catalysts for Electrochemical CO2 Reduction. Nano-Micro Lett. 2019, 11, 62. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Kang, P.; Meyer, T.J. Nanostructured Tin Catalysts for Selective Electrochemical Reduction of Carbon Dioxide to Formate. J. Am. Chem. Soc. 2014, 136, 1734–1737. [Google Scholar] [CrossRef]
- Bejtka, K.; Zeng, J.; Sacco, A.; Castellino, M.; Hernández, S.; Farkhondehfal, M.A.; Savino, U.; Ansaloni, S.; Pirri, C.F.; Chiodoni, A. Chainlike Mesoporous SnO2 as a Well-Performing Catalyst for Electrochemical CO2 Reduction. ACS Appl. Energy Mater. 2019, 2, 3081–3091. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Chen, L.; Xue, M.; Williams, T.; Zhang, Y.; MacFarlane, D.R.; Zhang, J. Towards a better Sn: Efficient electrocatalytic reduction of CO2 to formate by Sn/SnS2 derived from SnS2 nanosheets. Nano Energy 2017, 31, 270–277. [Google Scholar] [CrossRef]
- Choi, S.Y.; Jeong, S.K.; Kim, H.J.; Baek, I.-H.; Park, K.T. Electrochemical Reduction of Carbon Dioxide to Formate on Tin–Lead Alloys. ACS Sustain. Chem. Eng. 2016, 4, 1311–1318. [Google Scholar] [CrossRef]
- Min, X.; Kanan, M.W. Pd-Catalyzed Electrohydrogenation of Carbon Dioxide to Formate: High Mass Activity at Low Overpotential and Identification of the Deactivation Pathway. J. Am. Chem. Soc. 2015, 137, 4701–4708. [Google Scholar] [CrossRef]
- He, W.; Liberman, I.; Rozenberg, I.; Ifraemov, R.; Hod, I. Electrochemically Driven Cation Exchange Enables the Rational Design of Active CO2 Reduction Electrocatalysts. Angew. Chem. Int. Ed. 2020, 59, 8262–8269. [Google Scholar] [CrossRef]
- Deng, Y.; Huang, Y.; Ren, D.; Handoko, A.D.; Seh, Z.W.; Hirunsit, P.; Yeo, B.S. On the Role of Sulfur for the Selective Electrochemical Reduction of CO2 to Formate on CuSx Catalysts. ACS Appl. Mater. Interfaces 2018, 10, 28572–28581. [Google Scholar] [CrossRef]
- Gattrell, M.; Gupta, N.; Co, A. Electrochemical reduction of CO2 to hydrocarbons to store renewable electrical energy and upgrade biogas. Energy Convers. Manag. 2007, 48, 1255–1265. [Google Scholar] [CrossRef]
- Shinagawa, T.; Larrazábal, G.O.; Martín, A.J.; Krumeich, F.; Pérez-Ramírez, J. Sulfur-Modified Copper Catalysts for the Electrochemical Reduction of Carbon Dioxide to Formate. ACS Catal. 2018, 8, 837–844. [Google Scholar] [CrossRef] [Green Version]
- De Arquer, F.P.G.; Bushuyev, O.S.; De Luna, P.; Dinh, C.-T.; Seifitokaldani, A.; Saidaminov, M.I.; Tan, C.-S.; Na Quan, L.; Proppe, A.; Kibria, G.; et al. 2D Metal Oxyhalide-Derived Catalysts for Efficient CO2 Electroreduction. Adv. Mater. 2018, 30, e1802858. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Ni, F.; Ji, Y.; Wang, L.; Wen, Y.; Bai, H.; Liu, G.; Zhang, Y.; Li, Y.; Zhang, B.; et al. The p-Orbital Delocalization of Main-Group Metals to Boost CO2 Electroreduction. Angew. Chem. Int. Ed. 2018, 57, 16114–16119. [Google Scholar] [CrossRef]
- Yang, X.; Deng, P.; Liu, D.; Zhao, S.; Li, D.; Wu, H.; Ma, Y.; Xia, B.Y.; Li, M.; Xiao, C.; et al. Partial sulfuration-induced defect and interface tailoring on bismuth oxide for promoting electrocatalytic CO2 reduction. J. Mater. Chem. A 2020, 8, 2472–2480. [Google Scholar] [CrossRef]
- Koh, J.H.; Won, D.H.; Eom, T.; Kim, N.-K.; Jung, K.D.; Kim, H.; Hwang, Y.J.; Min, B.K. Facile CO2 Electro-Reduction to Formate via Oxygen Bidentate Intermediate Stabilized by High-Index Planes of Bi Dendrite Catalyst. ACS Catal. 2017, 7, 5071–5077. [Google Scholar] [CrossRef]
- Miao, C.-C.; Yuan, G.-Q. Morphology-Controlled Bi2O3 Nanoparticles as Catalysts for Selective Electrochemical Reduction of CO2 to Formate. ChemElectroChem 2018, 5, 3741–3747. [Google Scholar] [CrossRef]
- Liu, S.; Lu, X.F.; Xiao, J.; Wang, X.; Lou, X.W. Bi2O3 Nanosheets Grown on Multi-Channel Carbon Matrix to Catalyze Efficient CO2 Electroreduction to HCOOH. Angew. Chem. Int. Ed. 2019, 58, 13828–13833. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wang, H.; Qi, R.; Zhu, J.; Chen, S.; Yang, F.; Zhou, L.; Qi, K.; Liu, H.; Xia, B.Y. Bismuth Oxides with Enhanced Bismuth–Oxygen Structure for Efficient Electrochemical Reduction of Carbon Dioxide to Formate. ACS Catal. 2019, 10, 743–750. [Google Scholar] [CrossRef]
- Díaz-Sainz, G.; Alvarez-Guerra, M.; Solla-Gullón, J.; García-Cruz, L.; Montiel, V.; Irabien, A. CO2 electroreduction to formate: Continuous single-pass operation in a filter-press reactor at high current densities using Bi gas diffusion electrodes. J. CO2 Util. 2019, 34, 12–19. [Google Scholar] [CrossRef]
- Jagdale, P.; Ziegler, D.; Rovere, M.; Tulliani, J.M.; Tagliaferro, A.A. Waste Coffee Ground Biochar: A Material for Humidity Sensors. Sensors 2019, 19, 801. [Google Scholar] [CrossRef] [Green Version]
- Arrigo, R.; Jagdale, P.; Bartoli, M.; Tagliaferro, A.; Malucelli, G. Structure–Property Relationships in Polyethylene-Based Composites Filled with Biochar Derived from Waste Coffee Grounds. Polymers 2019, 11, 1336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carbon Monoxide Infrared Spectrum, (n.d.). Available online: https://spectrabase.com/spectrum/Big2BLmUPf2 (accessed on 16 March 2021).
- Carbon Dioxide Infrared Spectrum, (n.d.). Available online: https://spectrabase.com/spectrum/G4CBuKNSKtQ (accessed on 16 March 2021).
- Water Infrared Spectrum, (n.d.). Available online: https://spectrabase.com/spectrum/BnpcCEB1yUv (accessed on 16 March 2021).
- Gadhi, T.A.; Hernández, S.; Castellino, M.; Jagdale, P.; Husak, T.; Hernández-Gordillo, A.; Tagliaferro, A.; Russo, N. Insights on the role of β-Bi2O3/Bi5O7NO3 heterostructures synthesized by a scalable solid-state method for the sunlight-driven photocatalytic degradation of dyes. Catal. Today 2019, 321–322, 135–145. [Google Scholar] [CrossRef]
- Roumanille, P.; Baco-Carles, V.; Bonningue, C.; Gougeon, M.; Duployer, B.; Monfraix, P.; Le Trong, H.; Tailhades, P. Bi2(C2O4)3·7H2O and Bi(C2O4)OH Oxalates Thermal Decomposition Revisited. Formation of Nanoparticles with a Lower Melting Point than Bulk Bismuth. Inorg. Chem. 2017, 56, 9486–9496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Su, X.; Sun, Y.; Jin, L.; Zhang, L.; Yang, Y.; Kerns, P.; Liu, B.; Li, S.; He, J. Hierarchically porous Cu/Zn bimetallic catalysts for highly selective CO2 electroreduction to liquid C2 products. Appl. Catal. B Environ. 2020, 269, 118800. [Google Scholar] [CrossRef]
- Ju, W.; Bagger, A.; Hao, G.-P.; Varela, A.S.; Sinev, I.; Bon, V.; Cuenya, B.R.; Kaskel, S.; Rossmeisl, J.; Strasser, P. Understanding activity and selectivity of metal-nitrogen-doped carbon catalysts for electrochemical reduction of CO2. Nat. Commun. 2017, 8, 944. [Google Scholar] [CrossRef] [PubMed]
- Luc, W.; Collins, C.; Wang, S.; Xin, H.; He, K.; Kang, Y.; Jiao, F. Ag–Sn Bimetallic Catalyst with a Core–Shell Structure for CO2 Reduction. J. Am. Chem. Soc. 2017, 139, 1885–1893. [Google Scholar] [CrossRef] [PubMed]
- Tao, Z.; Wu, Z.; Yuan, X.; Wu, Y.; Wang, H. Copper–Gold Interactions Enhancing Formate Production from Electrochemical CO2 Reduction. ACS Catal. 2019, 9, 10894–10898. [Google Scholar] [CrossRef]








| Materials | Weight Loss (%) a | BiOx (wt.%) b | Biochar (wt.%) c | BiOx (wt.%) d |
|---|---|---|---|---|
| biochar | 87.4 | - | 100 | - |
| biochar–BiOx | 10.4 | 85.3 | 14.7 | 60.1–67% |
| Electrocatalyst | Electrolyte | Potential (V vs. RHE) | Current Density (-mA/cm2) | FE (%) | Reference |
|---|---|---|---|---|---|
| Biochar–BiOx | 2.0 M KHCO3 | −0.90 | 18.0 | 83 | This work |
| Biochar–BiOx | 2.0 M KHCO3 | −1.10 | 52.4 | 87 | This work |
| Bi Dendrite | 0.5 M KHCO3 | −0.74 | 2.7 | 89 | [31] |
| Bi Dendrite | 0.5 M KHCO3 | −1.14 | 41.0 | 38 | [31] |
| Bi2O3 Nanoparticles | 0.5 M NaHCO3 | −1.00 | - | 82 | [32] |
| Bi2O3 Nanoparticles | 0.5 M NaHCO3 | −1.20 | 20.0 | 91 | [32] |
| Bi2O3 Nanosheets | 0.1 M KHCO3 | −0.86 | 1.9 | 65 | [33] |
| Bi2O3 Nanosheets | 0.1 M KHCO3 | −1.06 | 7.8 | 97 | [33] |
| Bi2O3 | 0.5 M KHCO3 | −0.90 | 8.0 | 91 | [34] |
| Bi2O3 | 0.5 M KHCO3 | −1.10 | - | 72 | [34] |
| nano-SnO2/C | 0.1 M NaHCO3 | −1.16 | 6.2 | 86 | [19] |
| SnO2 | 0.1 M KHCO3 | −1.06 | 11.0 | 82 | [20] |
| Cu2S | 0.1 M KHCO3 | −0.90 | 19.0 | 87 | [24] |
| CuSx | 0.1 M KHCO3 | −0.85 | 11.9 | 75 | [25] |
| Cu1.81S | 0.5 M KHCO3 | −0.67 | 3.8 | 82 | [26] |
| S-modified Cu | 0.1 M KHCO3 | −0.80 | 20.0 | 80 | [27] |
| SnS2/RGO | 0.5 M NaHCO3 | −1.40 (vs. Ag/AgCl) | 13.9 | 84 | [21] |
| Bi2S3–Bi2O3@rGO | 0.1 M KHCO3 | −0.90 | 3.8 | 90 | [30] |
| Sn56.3Pb43.7 | 0.5 M KHCO3 | −2.00 (vs. Ag/AgCl) | 45.7 | 80 | [22] |
| AgSn/SnOx | 0.5 M NaHCO3 | −0.80 | 16.0 | 80 | [45] |
| Cu-Au | 0.5M KHCO3 | −0.60 | 10.2 | 81 | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, J.; Jagdale, P.; Lourenço, M.A.O.; Farkhondehfal, M.A.; Sassone, D.; Bartoli, M.; Pirri, C.F. Biochar-Supported BiOx for Effective Electrosynthesis of Formic Acid from Carbon Dioxide Reduction. Crystals 2021, 11, 363. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040363
Zeng J, Jagdale P, Lourenço MAO, Farkhondehfal MA, Sassone D, Bartoli M, Pirri CF. Biochar-Supported BiOx for Effective Electrosynthesis of Formic Acid from Carbon Dioxide Reduction. Crystals. 2021; 11(4):363. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040363
Chicago/Turabian StyleZeng, Juqin, Pravin Jagdale, Mirtha A. O. Lourenço, M. Amin Farkhondehfal, Daniele Sassone, Mattia Bartoli, and Candido Fabrizio Pirri. 2021. "Biochar-Supported BiOx for Effective Electrosynthesis of Formic Acid from Carbon Dioxide Reduction" Crystals 11, no. 4: 363. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11040363