Carbonation of Sodium Aluminate/Sodium Carbonate Solutions for Precipitation of Alumina Hydrates—Avoiding Dawsonite Formation
Abstract
:1. Introduction
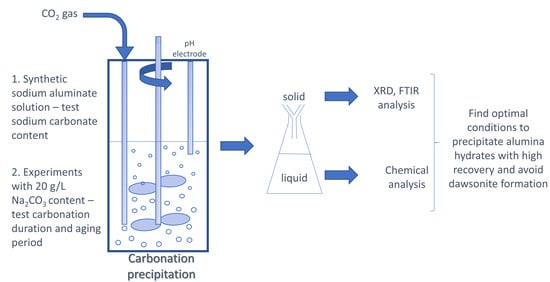
2. Materials and Methods
3. Results
3.1. Effect of Initial Sodium Carbonate Concentration
3.2. Effect of Carbonation Duration
3.3. Effect of Aging
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Balomenos, E.; Davris, P.; Pontikes, Y.P.D.; Delipaltas, A. Bauxite Residue Handling Practice and Valorisation Research in Aluminium of Greece. In Proceedings of the 2nd International Bauxite Residue Valorisation and Best Practices Conference, Athens, Greece, 7–10 May 2018; pp. 29–38. [Google Scholar]
- Miller, J.; Irgens, A. Alumina Production by the Pedersen Process—History and Future. In Essential Readings in Light Metals; Donaldson, D., Raahauge, B.E., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 977–982. [Google Scholar]
- Hosteman, J.W.; Patterson, S.H.; Good, E.E. World Nonbauxite Aluminum Resources Excluding Alunite; 1076C; United States Department of Energy: Washington, DC, USA, 1990.
- Vafeias, M.; Marinos, D.; Panias, D.; Safarian, J.; Van Der Eijk, C.; Solhem, I.; Davris, P. From red to grey: Revisiting the Pedersen process to achieve holistic bauxite ore utilisation. In Proceedings of the 2nd International Bauxite Residue Valorisation and Best Practices Conference, Athens, Greece, 7–10 May 2018; pp. 111–117. [Google Scholar]
- Nielsen, K. The Pedersen Process—An old process in a new light. Erzmetall 1978, 31, 523–525. [Google Scholar]
- Barr, L.F.K. Alumina production from andalusite by the Pedersen process. Trans. Inst. Min. Metall. Sect. C Miner. Process. Extr. Metall. 1977, 86, C64–C70. [Google Scholar]
- Lazou, A.; Van Der Eijk, C.; Tang, K.; Balomenos, E.; Kolbeinsen, L.; Safarian, J. The Utilization of Bauxite Residue with a Calcite-Rich Bauxite Ore in the Pedersen Process for Iron and Alumina Extraction. Metall. Mater. Trans. B 2021, 52, 1255–1266. [Google Scholar] [CrossRef]
- Hignett, T. PILOT PLANTS: Production of Alumina from Clay by a Modified Pedersen Process. Ind. Eng. Chem. 1947, 39, 1052–1060. [Google Scholar] [CrossRef]
- Pedersen, H. Process of Manufacturing Aluminum Hydroxide. U.S. Patent 1,618,105, 15 February 1927. [Google Scholar]
- Aktieselskapet Norsk Aluminium. Process for the Manufacture of Aluminium Oxide. U.S. Patent 252,399, 9 June 1927.
- Peter William Reynolds Laurence Roy Pittwell Ici Ltd. Improvements in and Relating to the Production of Alumina. U.S. Patent GB-667145-A, 26 October 1949.
- Thomson, M.R.; McLeod, H.M.J.; Skow, M.L. Recovery of Alumina from Submarginal Bauxites—Part. 2.—Extraction of Alumina from Electric-Furnace Slags of Calcium Aluminate; United States Department of the Interior-Bureau of Mines: Washington, DC, USA, 1949.
- Copson, R.L.; Walthall, J.H.; Hignett, T.P. Aluminum-Extraction of Alumina from Clays by the Lime-Sinter Modification of the Pedersen Process; The American Institute of Mining, Metallurgical, and Petroleum Engineers: New York, NY, USA, 1944. [Google Scholar]
- Shayanfar, S.; Aghazadeh, V.; Saravari, A.; Hasanpour, P. Aluminum hydroxide crystallization from aluminate solution using carbon dioxide gas: Effect of temperature and time. J. Cryst. Growth 2018, 496, 1–9. [Google Scholar] [CrossRef]
- Aghazadeh, V.; Shayanfar, S.; Samiee Beyragh, A. Thermodynamic Modeling and Experimental Studies of Bayerite Precipitation from Aluminate Solution: Temperature and pH Effect. Iran. J. Chem. Chem. Eng. 2019, 38, 229–238. [Google Scholar] [CrossRef]
- Aghazadeh, V.; Shayanfar, S.; Hassanpour, P. Aluminum hydroxide crystallization from aluminate solution using carbon dioxide gas: Effect of pH and seeding. Miner. Process. Extr. Metall. 2019. [Google Scholar] [CrossRef]
- Blake, H.E. Adaptation of the Pedersen Process to the Ferruginous Bauxites of the Pacific Northwest; Department of the Interior, Bureau of Mines: Washington, DC, USA, 1967.
- Wang, Z.; Yang, L.; Zhang, J.; Guo, Z.-C.; Zhang, Y. Adjustment on gibbsite and boehmite co-precipitation from supersaturated sodium aluminate solutions. Trans. Nonferrous Met. Soc. 2010, 20, 521–527. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Y.; Yang, C.; Zhang, Y. Precipitating sandy aluminium hydroxide from sodium aluminate solution by the neutralization of sodium bicarbonate. Hydrometallurgy 2009, 98, 52–57. [Google Scholar] [CrossRef]
- Panov, A.; Senyuta, A.; Smirnov, A.; Pechenkin, M. Revisiting alternative smelter grade alumina production processes. In Proceedings of the TMS 2021, Virtual, Pittsburgh, PA, USA, 15–18 March 2021. [Google Scholar]
- Safarian, J.; Kolbeinsen, L. Sustainability in alumina production from bauxite. In Proceedings of the 2016 Sustainable Industrial Processing Summit and Exhibition, Flogen, Hainan-Island, China, 6–10 November 2016. [Google Scholar]
- Padilla, R.; Sohn, H.Y. Sodium aluminate leaching and desilication in lime-soda sinter process for alumina from coal wastes. Metall. Trans. B 1985, 16, 707–713. [Google Scholar] [CrossRef]
- Li, Y.; Lei, T.; Yang, D.J. Radionuclide of Process of Carbon Decomposition and Anneal of Liquor after Desilication from Nepheline. Appl. Mech. Mater. 2013, 330, 22–26. [Google Scholar] [CrossRef]
- Wojcik, M.; Pyzalski, M. Factors effecting on the properties of bayerite obtained in the carbonization process of alkaline aluminate solutions. In Proceedings of the Light Metals 1990 AIME Annual Meeting, Anaheim, CA, USA, 18–22 February 1990; pp. 161–165. [Google Scholar]
- Zhou, Q.; Peng, D.; Peng, Z.; Liu, G.; Li, X. Agglomeration of gibbsite particles from carbonation process of sodium aluminate solution. Hydrometallurgy 2009, 99, 163–169. [Google Scholar] [CrossRef]
- Lee, M.-Y.; Parkinson, G.M.; Smith, P.G.; Lincoln, F.J.; Reyhani, M.M. Characterization of Aluminum Trihydroxide Crystals Precipitated from Caustic Solutions. In Separation and Purification by Crystallization; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1997; Volume 667, pp. 123–133. [Google Scholar]
- Stewart, J.J. Comparison of the accuracy of semiempirical and some DFT methods for predicting heats of formation. J. Mol. Model. 2004, 10, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Tettenhorst, R. Crystal Chemistry of Boehmite. Clays Clay Miner. 1980, 28, 373–380. [Google Scholar] [CrossRef]
- Sato, T.; Sato, K. Preparation of Gelatinous Aluminium Hydroxide from Aqueous Solutions of Aluminium Salts Containing Sulphate Group with Alkali. J. Ceram. Soc. Jpn. 1996, 104, 377–382. [Google Scholar] [CrossRef] [Green Version]
- Nail, S.L.; White, J.L.; Hem, S.L. Comparison of IR spectroscopic analysis and X-ray diffraction of aluminum hydroxide gel. J. Pharm. Sci 1975, 64, 1166–1169. [Google Scholar] [CrossRef]
- Bardossy, G.; White, J.L. Carbonate inhibits the crystallization of aluminum hydroxide in bauxite. Science 1979, 203, 355–356. [Google Scholar] [CrossRef]
- Serna, C.J.; Lyons, J.C.; White, J.L.; Hem, S.L. Stabilization of aluminum hydroxide gel by specifically adsorbed carbonate. J. Pharm. Sci 1983, 72, 769–771. [Google Scholar] [CrossRef]
- Koga, N.; Fukagawa, T.; Tanaka, H. Preparation and Thermal Decomposition of Synthetic Bayerite. J. Therm. Anal. Calorim. 2001, 64, 965–972. [Google Scholar] [CrossRef]
- Balan, E.; Blanchard, M.; Hochepied, J.-F.; Lazzeri, M. Surface modes in the infrared spectrum of hydrous minerals: The OH stretching modes of bayerite. Phys. Chem. Miner. 2008, 35, 279–285. [Google Scholar] [CrossRef]
- Wolska, E.; Szajda, W. Use of infrared spectroscopy to identify crystalline aluminum hydroxides of the Al(OH)3-Fe(OH)3 system. J. Appl. Spectrosc. 1983, 38, 137–140. [Google Scholar] [CrossRef]
- Sato, T.; Yamashita, T.; Ozawa, F. The Preparation of Bayerite from sodium aluminate solutions with carbon dioxide. Z. Für Anorg. Allg. Chem. 1969, 370, 202–208. [Google Scholar] [CrossRef]
- Jraba, N.; Tounsi, H.; Makhlouf, T. Valorization of Aluminum Chips into γ-Al2O3 and η-Al2O3 with High Surface Areas via the Precipitation Route. Waste Biomass Valorization 2016, 9, 1003–1014. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Rezai, B.; Bahri, Z.; Mahmoudian, M. Influences of New Synthesized Active Seeds and Industrial Seed on the Aluminum Hydroxide Precipitation from Sodium Aluminate Solution. J. Sustain. Metall. 2020, 6, 643–658. [Google Scholar] [CrossRef]
- Jodin-Caumon, M.C.; Humbert, B.; Phambu, N.; Gaboriaud, F. A vibrational study of the nature of hydroxyl groups chemical bonding in two aluminium hydroxides. Spectrochim Acta A Mol. Biomol. Spectrosc. 2009, 72, 959–964. [Google Scholar] [CrossRef] [PubMed]
- White, J.L.; Hem, S.L. Role of carbonate in aluminum hydroxide gel established by Raman and IR analyses. J. Pharm. Sci. 1975, 64, 468–469. [Google Scholar] [CrossRef] [PubMed]
- Le Bozec, N.; Persson, D.; Nazarov, A.; Thierry, D. Investigation of Filiform Corrosion on Coated Aluminum Alloys by FTIR Microspectroscopy and Scanning Kelvin Probe. J. Electrochem. Soc. 2002, 149, B403–B408. [Google Scholar] [CrossRef]
- Kiss, A.B.; Keresztury, G.; Farkas, L. Raman and i.r. spectra and structure of boehmite (γ-AlOOH). Evidence for the recently discarded D172h space group. Spectrochim. Acta Part A Mol. Spectrosc. 1980, 36, 653–658. [Google Scholar] [CrossRef]
- Russell, J.D.; Farmer, V.C.; Lewis, D.G. Lattice vibrations of boehmite (γ-AlOOH): Evidence for a C122v rather than a D172h space group. Spectrochim. Acta Part A Mol. Spectrosc. 1978, 34, 1151–1153. [Google Scholar] [CrossRef]
- Alex, T.C.; Kailath, A.J.; Kumar, R. Al-Monohydrate (Boehmite) to Al-Trihydrate (Bayerite/Gibbsite) Transformation during High-Energy Milling. Metall. Mater. Trans. B 2020, 51, 443–451. [Google Scholar] [CrossRef]
- Sato, T. Preparation of aluminium hydroxide by reacting sodium aluminate solutions with mineral acid. J. Chem. Technol. Biotechnol. 2007, 31, 670–675. [Google Scholar] [CrossRef]
- Panias, D.; Krestou, A. Effect of synthesis parameters on precipitation of nanocrystalline boehmite from aluminate solutions. Powder Technol. 2007, 175, 163–173. [Google Scholar] [CrossRef]
- Panias, D.; Paspaliaris, I. Precipitation of Boehmite—An Innovative Route in the Alumina Production; National Technical University of Athens: Athens, Greece, 1998. [Google Scholar]
- Czajkowski, A.; Noworyta, A.; Krótki, M. Studies and modelling of the process of decomposition of aluminate solutions by carbonation. Hydrometallurgy 1981, 7, 253–261. [Google Scholar] [CrossRef]
- Krestou, A.; Panias, D. Alumina Hydrate Precipitates in the System NaAl(OH)4 (Supersaturated)/HNO3; National Technical University of Athens: Athens, Greece, 2005; Volume 4. [Google Scholar]
- Bradley, S.M.; Kydd, R.A.; Howe, R.F. The Structure of Al Gels Formed through the Base Hydrolysis of Al3+ Aqueous Solutions. J. Colloid Interface Sci. 1993, 159, 405–412. [Google Scholar] [CrossRef]
- Li, H.; Addai-Mensah, J.; Thomas, J.C.; Gerson, A.R. The crystallization mechanism of Al(OH)3 from sodium aluminate solutions. J. Cryst. Growth 2005, 279, 508–520. [Google Scholar] [CrossRef]
- Gates-Rector, S.; Blanton, T. The Powder Diffraction File: A quality materials characterization database. Powder Diffr. 2019, 34, 352–360. [Google Scholar] [CrossRef] [Green Version]









| Compound | Starting Synthetic Solution to Precipitation (g/L) |
|---|---|
| Na2CO3 | 20, 40, 60, 80, 160 |
| NaOH | 18.75 |
| Al | 8–8.5 |
| Si | 0.2–0.24 |
| Attributed Phase | Wavenumber (cm−1) | Functional Group/Vibration | References |
|---|---|---|---|
| Bayerite | 3656, 3550, 3462, 3438, 3429 | O–H stretching band | [33,34,35,36,37,38,39] |
| 1021, 981 | O–H bending band | [33,35,36,37] | |
| 718 | Al–OH vibration | [33,36] | |
| Aluminum hydroxide carbonate gel | 1523, 1406 | CO3−2 asymmetric stretching band | [32,40,41] |
| Boehmite | 3316, 3096 | O–H stretching band | [32,35,37,42,43,44] |
| Adsorbed water | 1651 | H–O–H bending | [30,37,38,44,45] |
| Region | Phase Precipitating | Maximum Al Recovery (%) in Precipitate after Aging | Range of Dph/Dt |
|---|---|---|---|
| I | Sodium zeolite | 10% | 0 to −0.03 |
| II | Alumina hydrates | 58% | −0.04 to −0.15 |
| III | Alumina hydrates | 84% | 0 to −0.01 |
| IV | Metastable alumina hydrates | 94% | 0.01 to 0.02 |
| V | Dawsonite | 100% | 0 to −0.02 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marinos, D.; Kotsanis, D.; Alexandri, A.; Balomenos, E.; Panias, D. Carbonation of Sodium Aluminate/Sodium Carbonate Solutions for Precipitation of Alumina Hydrates—Avoiding Dawsonite Formation. Crystals 2021, 11, 836. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070836
Marinos D, Kotsanis D, Alexandri A, Balomenos E, Panias D. Carbonation of Sodium Aluminate/Sodium Carbonate Solutions for Precipitation of Alumina Hydrates—Avoiding Dawsonite Formation. Crystals. 2021; 11(7):836. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070836
Chicago/Turabian StyleMarinos, Danai, Dimitrios Kotsanis, Alexandra Alexandri, Efthymios Balomenos, and Dimitrios Panias. 2021. "Carbonation of Sodium Aluminate/Sodium Carbonate Solutions for Precipitation of Alumina Hydrates—Avoiding Dawsonite Formation" Crystals 11, no. 7: 836. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11070836