Physicochemical, Photocatalytic, Antibacterial, and Antioxidant Screening of Bergenia Ciliata Mediated Nickel Oxide Nanoparticles
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemical Used
2.2. Preparation of Plant Extract
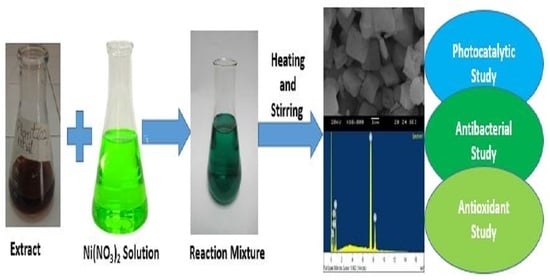
2.3. Green Synthesis of NiO NPs
2.4. Physicochemical Characterization
2.5. Antibacterial Assay
2.6. Antioxidant Assay
3. Results and Discussion
3.1. Physicochemical Study
3.1.1. XRD Analysis
3.1.2. Microstructure Analysis
3.1.3. UV-Visible and EDX Analysis
3.1.4. FTIR Analysis
3.2. Photocatalytic Activity
3.3. Antibacterial Activity
3.4. Antioxidant Activity
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Barzinjy, A.A.; Hamad, S.M.; Aydın, S.; Ahmed, M.H.; Hussain, F.H.S. Green and eco-friendly synthesis of Nickel oxide nanoparticles and its photocatalytic activity for methyl orange degradation. J. Mater. Sci. Mater. Electron. 2020, 31, 11303–11316. [Google Scholar] [CrossRef]
- Br, S.; Xr, J. Effect of calcination time on structural, optical and antimicrobial properties of nickel oxide nanoparticles. Theor. Comput. Sci. 2016, 3, 149–159. [Google Scholar] [CrossRef] [Green Version]
- El-Kemary, M.; Nagy, N.; El-Mehasseb, I. Nickel oxide nanoparticles: Synthesis and spectral studies of interactions with glucose. Mater. Sci. Semicond. Process. 2013, 16, 1747–1752. [Google Scholar] [CrossRef]
- Suresh, K.C.; Balamurugan, A. Evaluation of structural, optical, and morphological properties of nickel oxide nanoparticles for multi-functional applications. Inorg. Nano-Metal. Chem. 2020, 51, 296–301. [Google Scholar] [CrossRef]
- Lingaraju, K.; Raja Naika, H.; Nagabhushana, H.; Jayanna, K.; Devaraja, S.; Nagaraju, G. Biosynthesis of nickel oxide nanoparticles from euphorbia heterophylla (L.) and their biological application. Arab. J. Chem. 2020, 13, 4712–4719. [Google Scholar] [CrossRef]
- Ezhilarasi, A.A.; Vijaya, J.J.; Kaviyarasu, K.; Zhang, X.; Kennedy, L.J. Green synthesis of nickel oxide nanoparticles using solanum trilobatum extract for cytotoxicity, antibacterial and photocatalytic studies. Surf. Interfaces 2020, 20, 100553. [Google Scholar] [CrossRef]
- Feiona, T.A.; Sabeena, G.; Bagavathy, M.S.; Pushpalaksmi, E.; Samraj, J.J. Recent advances in the synthesis and characterization of nanoparticles: A green adeptness for photocatalytic and antibacterial activity. Nat. Environ. Pollut. Technol. 2021, 20, 657–663. [Google Scholar] [CrossRef]
- Mokoena, T.P.; Swart, H.C.; Motaung, D.E. A review on recent progress of p-type nickel oxide based gas sensors: Future perspectives. J. Alloys Compd. 2019, 805, 267–294. [Google Scholar] [CrossRef]
- Arora, A.K.; Jaswal, V.S.; Singh, K.; Singh, R. Applications of metal/mixed metal oxides as photocatalyst: A review. Orient. J. Chem. 2016, 32, 2035–2042. [Google Scholar] [CrossRef]
- Niklasson, G.A.; Granqvist, C.G. Electrochromics for smart windows: Thin films of tungsten oxide and nickel oxide, and devices based on these. J. Mater. Chem. 2007, 17, 127–156. [Google Scholar] [CrossRef] [Green Version]
- Danjumma, S.G.; Abubakar, Y.; Suleiman, S. Nickel Oxide (NiO) devices and applications: A review. Int. J. Eng. Res. Technol. 2019, V8, 461–467. [Google Scholar] [CrossRef]
- Hameed, A.; Gombac, V.; Montini, T.; Graziani, M.; Fornasiero, P. Synthesis, Characterization and photocatalytic activity of NiO–Bi2O3 nanocomposites. Chem. Phys. Lett. 2009, 472, 212–216. [Google Scholar] [CrossRef]
- Haq, S.; Dildar, S.; Ali, M.B.; Mezni, A.; Hedfi, A.; Shahzad, M.I.; Shahzad, N.; Shah, A. Antimicrobial and antioxidant properties of biosynthesized of NiO nanoparticles using raphanus sativus (R. sativus) extract. Mater. Res. Express 2021, 8, 055006. [Google Scholar] [CrossRef]
- Sone, B.T.; Fuku, X.G.; Maaza, M. Physical & electrochemical properties of green synthesized bunsenite NiO nanoparticles via callistemon viminalis’ extracts. Int. J. Electrochem. Sci. 2016, 11, 8204–8220. [Google Scholar] [CrossRef]
- Angel Ezhilarasi, A.; Judith Vijaya, J.; Kaviyarasu, K.; John Kennedy, L.; Ramalingam, R.J.; Al-Lohedan, H.A. Green synthesis of NiO nanoparticles using aegle marmelos leaf extract for the evaluation of in-vitro cytotoxicity, antibacterial and photocatalytic properties. J. Photochem. Photobiol. B Biol. 2018, 180, 39–50. [Google Scholar] [CrossRef]
- Anand, G.T.; Nithiyavathi, R.; Ramesh, R.; John Sundaram, S.; Kaviyarasu, K. Structural and optical properties of nickel oxide nanoparticles: Investigation of antimicrobial applications. Surf. Interfaces 2020, 18, 100460. [Google Scholar] [CrossRef]
- Sinha, S.; Murugesan, T.; Maiti, K.; Gayen, J.R.; Pal, M.; Saha, B.P. Evaluation of anti-inflammatory potential of bergenia ciliata sternb. rhizome extract in rats. J. Pharm. Pharmacol. 2010, 53, 193–196. [Google Scholar] [CrossRef]
- Hamid, A.; Haq, S.; Ur Rehman, S.; Akhter, K.; Rehman, W.; Waseem, M.; Ud Din, S.U.; Zain-ul-Abdin; Hafeez, M.; Khan, A.; et al. Calcination temperature-driven antibacterial and antioxidant activities of fumaria indica mediated copper oxide nanoparticles: Characterization. Chem. Pap. 2021, 75, 4189–4198. [Google Scholar] [CrossRef]
- Sagadevan, S.; Podder, J. Investigations on structural, optical, morphological and electrical properties of nickel oxide nanoparticles. Int. J. Nanoparticles 2015, 8, 289–301. [Google Scholar] [CrossRef]
- Goel, R.; Jha, R.; Ravikant, C. Investigating the structural, electrochemical, and optical properties of p-type spherical nickel oxide (NiO) nanoparticles. J. Phys. Chem. Solids 2020, 144, 109488. [Google Scholar] [CrossRef]
- Shah, A.; Haq, S.; Rehman, W.; Muhammad, W.; Shoukat, S.; Rehman, M. Photocatalytic and antibacterial activities of paeonia emodi mediated silver oxide nanoparticles. Mater. Res. Express 2019, 6, 045045. [Google Scholar] [CrossRef]
- Ahmad, S.; Fahmina, B.; Aftab, Z.; Mondal, H.; Kareem, A.; Ullah, A. Photocatalytic degradation of carcinogenic congo red dye in aqueous solution, antioxidant activity and bactericidal effect of NiO nanoparticles. J. Iran. Chem. Soc. 2020, 17, 215–227. [Google Scholar] [CrossRef]
- Shoukat, S.; Rehman, W.; Haq, S.; Waseem, M.; Shah, A. Synthesis and characterization of zinc stannate nanostructures for the adsorption of chromium (VI) ions and photo-degradation of rhodamine 6G. Mater. Res. Express 2019, 6, 115052. [Google Scholar] [CrossRef]
- Haq, S.; Rehman, W.; Waseem, M.; Meynen, V.; Awan, S.U.; Khan, A.R.; Hussain, S.; Zain-ul-Abdin; Din, S.U.; Hafeez, M.; et al. Effect of annealing temperature on structural phase transformations and band gap reduction for photocatalytic activity of mesopores TiO2 nanocatalysts. J. Inorg. Organomet. Polym. Mater. 2021, 31, 1312–1322. [Google Scholar] [CrossRef]
- Haq, S.; Yasin, K.A.; Rehman, W.; Waseem, M.; Ahmed, M.N.; Shahzad, M.I.; Shahzad, N.; Shah, A.; Rehman, M.U.; Khan, B. Green synthesis of silver oxide nanostructures and investigation of their synergistic effect with moxifloxacin against selected microorganisms. J. Inorg. Organomet. Polym. Mater. 2020, 31, 1134–1142. [Google Scholar] [CrossRef]
- Haq, S.; Ahmad, P.; Khandaker, M.U.; Faruque, M.R.I.; Rehman, W.; Waseem, M.; Din, S.U. Antibacterial, antioxidant and physicochemical investigations of tin dioxide nanoparticles synthesized via microemulsion method. Mater. Res. Express 2021, 8, 035013. [Google Scholar] [CrossRef]
- Shah, A.; Tauseef, I.; Ali, M.B.; Yameen, M.A.; Mezni, A.; Hedfi, A.; Haleem, S.K.; Haq, S. In-vitro and in-vivo tolerance and therapeutic investigations of phyto-fabricated iron oxide nanoparticles against selected pathogens. Toxics 2021, 9, 105. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Haq, S.; Abbasi, F.; Ben Ali, M.; Hedfi, A.; Mezni, A.; Rehman, W.; Waseem, M.; Khan, A.R.; Shaheen, H. Green synthesis of cobalt oxide nanoparticles and the effect of annealing temperature on their physiochemical and biological properties. Mater. Res. Express 2021, 8, 075009. [Google Scholar] [CrossRef]







| Bacteria | Zones of Inhibition Measured in Millimeter (mm) with Error of ±2 | |||||
|---|---|---|---|---|---|---|
| 5 mg/5 mL | 15 mg/5 mL | 25 mg/5 mL | 50 mg/5 mL | PC | Solvent | |
| E. coli | 1.87 | 3.93 | 8.63 | 13.09 | 14.72 | 00 |
| S. aureus | 00 | 00 | 4.01 | 10.29 | 12.37 | 00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rehman, F.U.; Mahmood, R.; Ali, M.B.; Hedfi, A.; Mezni, A.; Haq, S.; Din, S.U.; Ehsan, R. Physicochemical, Photocatalytic, Antibacterial, and Antioxidant Screening of Bergenia Ciliata Mediated Nickel Oxide Nanoparticles. Crystals 2021, 11, 1137. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091137
Rehman FU, Mahmood R, Ali MB, Hedfi A, Mezni A, Haq S, Din SU, Ehsan R. Physicochemical, Photocatalytic, Antibacterial, and Antioxidant Screening of Bergenia Ciliata Mediated Nickel Oxide Nanoparticles. Crystals. 2021; 11(9):1137. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091137
Chicago/Turabian StyleRehman, Fazal Ur, Rashid Mahmood, Manel Ben Ali, Amor Hedfi, Amine Mezni, Sirajul Haq, Salah Ud Din, and Rimsha Ehsan. 2021. "Physicochemical, Photocatalytic, Antibacterial, and Antioxidant Screening of Bergenia Ciliata Mediated Nickel Oxide Nanoparticles" Crystals 11, no. 9: 1137. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst11091137