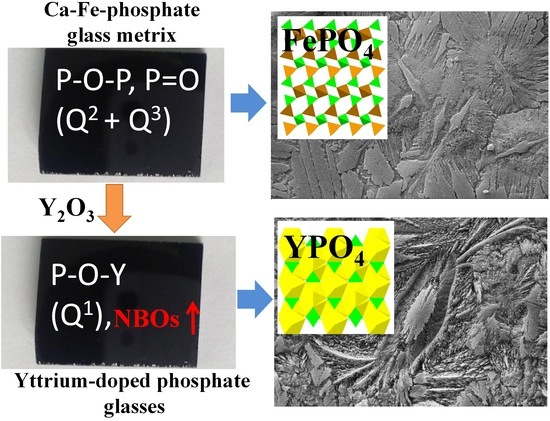
Spectroscopic and Surface Crystallization Characterizations of Yttrium-Doped Phosphate Glasses
Abstract
:1. Introduction
2. Experimental Procedure
2.1. Preparation of Glass and Glass-Ceramic Samples
2.2. Characterization Techniques
3. Results and Discussion
3.1. Composition and Structure of Glasses
3.2. Surface Crystallization Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pavić, L.; Skoko, Ž.; Gajović, A.; Su, D.S.; Moguš-Milanković, A. Electrical transport in iron phosphate glass-ceramics. J. Non-Cryst. Solids 2018, 502, 44–53. [Google Scholar] [CrossRef]
- Shi, Q.S.; Yue, Y.L.; Qu, Y.; Liu, S.Q.; Khater, G.A.; Zhang, L.L.; Zhao, J.L.; Kang, J.F. Structure and chemical durability of calcium iron phosphate glasses doped with La2O3 and CeO2. J. Non-Cryst. Solids 2019, 516, 50–55. [Google Scholar] [CrossRef]
- Qian, B.; Liang, X.F.; Wang, C.L.; Yang, S.Y. Structure and properties of calcium iron phosphate glasses. J. Nucl. Mater. 2013, 443, 140–144. [Google Scholar] [CrossRef]
- Li, H.J.; Liang, X.F.; Yu, H.J.; Yang, D.Q.; Yang, S.Y. Studies of structure of calcium–iron phosphate glasses by infrared, Raman and UV–Vis spectroscopies. Indian J. Phys. 2015, 90, 693–698. [Google Scholar] [CrossRef]
- Danilov, S.S.; Stefanovsky, S.V.; Stefanovskaya, O.I.; Vinokurov, S.E.; Myasoedov, B.F.; Teterin, Y.A. Aluminum (Iron) Phosphate Glasses Containing Rare Earth and Transuranium Elements: Phase Composition, Oxidation State of Np and Pu, and Hydrolytic Durability. Radiochemistry 2018, 60, 434–439. [Google Scholar] [CrossRef]
- Liang, X.F.; Li, H.J.; Wang, C.L.; Yu, H.J.; Li, Z.; Yang, S.Y. Physical and structural properties of calcium iron phosphate glass doped with rare earth. J. Non-Cryst. Solids 2014, 402, 135–140. [Google Scholar] [CrossRef]
- Wang, Y.L.; Wang, F.; Wang, Q.; Zhu, H.Z.; Xiang, G.H.; Liao, Q.L.; Zhu, Y.C. Effect of neodymium on the glass formation, dissolution rate and crystallization kinetic of borophosphate glasses containing iron. J. Non-Cryst. Solids 2019, 526, 119726. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovsky, O.I.; Kadyko, M.I.; Nikonov, B.S. Sodium aluminum-iron phosphate glass-ceramics for immobilization of lanthanide oxide wastes from pyrochemical reprocessing of spent nuclear fuel. J. Nucl. Mater. 2018, 500, 153–165. [Google Scholar] [CrossRef]
- He, Y.; Lü, Y.J.; Zhang, Q. Characterization of monazite glass–ceramics as wasteform for simulated α-HLLW. J. Nucl. Mater. 2008, 376, 201–206. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Stefanovsky, O.I.; Danilov, S.S.; Kadyko, M.I. Phosphate-based glasses and glass ceramics for immobilization of lanthanides and actinides. Ceram. Int. 2019, 45, 9331–9338. [Google Scholar] [CrossRef]
- Rodríguez-López, S.; Pascual, M.J. Sintering/Crystallization and Viscosity of Sealing Glass-Ceramics. Crystals 2021, 11, 737. [Google Scholar] [CrossRef]
- Maeda, K.; Akatsuka, K.; Okuma, G.; Yasumori, A. Mechanical Properties of CaO–Al2O3–SiO2 Glass-Ceramics Precipitating Hexagonal CaAl2Si2O8 Crystals. Crystals 2021, 11, 393. [Google Scholar] [CrossRef]
- Lai, F.; Leng, M.; Li, J.; Liu, Q. The Crystallization Behaviors of SiO2-Al2O3-CaO-MgO-TiO2 Glass-Ceramic Systems. Crystals 2020, 10, 794. [Google Scholar] [CrossRef]
- Hsu, J.H.; Bai, J.C.; Kim, C.W.; Brow, R.K.; Szabo, J.; Zervos, A. The effects of crystallization and residual glass on the chemical durability of iron phosphate waste forms containing 40 wt% of a high MoO3 Collins-CLT waste. J. Nucl. Mater. 2018, 500, 373–380. [Google Scholar] [CrossRef]
- Morales, A.E.; Mora, E.S.; Pal, U. Use of diffuse reflectance spectroscopy for optical characterization of un-supported nanostructures. Rev. Mexic. Física S 2007, 53, 18–22. [Google Scholar]
- Vance, E.R.; Zhang, Y.; McLeod, T.; Davis, J. Actinide valences in xenotime and monazite. J. Nucl. Mater. 2011, 409, 221–224. [Google Scholar] [CrossRef]
- Stefanovsky, S.V.; Prusakov, I.L.; Stefanovsky, O.I.; Kadyko, M.I.; Averin, A.A.; Makarenkov, V.I.; Trigub, A.L.; Nikonov, B.S. The structure of rhenium-containing sodium alumino (iron) phosphate glasses. Int. J. Appl. Glass Sci. 2019, 10, 479–487. [Google Scholar] [CrossRef]
- Qian, B.; Yang, S.Y.; Liang, X.F.; Lai, Y.M.; Gao, L.; Yin, G.F. Structural and thermal properties of La2O3-Fe2O3-P2O5 glasses. J. Mol. Struct. 2012, 1011, 153–157. [Google Scholar] [CrossRef]
- Łagowska, B.; Wacławska, I.; Sitarz, M.; Szumera, M. Spectroscopic studies of structural interactions in silicate-borate-phosphate glass. J. Mol. Struct. 2018, 1171, 110–116. [Google Scholar] [CrossRef]
- Cole, J.M.; Eck, E.R.H.V.; Mountjoy, G.; Anderson, R.; Brennan, T.; Bushnell-Wye, G.; Newport, R.J.; Saunders, G.A. An x-ray diffraction and 31P MAS NMR study of rare-earth phosphate glasses, (R2O3)x(P2O5)1-x, x = 0.175–0.263, R = La, Ce, Pr, Nd, Sm, Eu, Gd, Tb, Dy, Ho, Er. J. Phys. Condens Mat. 2001, 13, 4105. [Google Scholar] [CrossRef] [Green Version]
- Hoppe, U.; Brow, R.K.; Ilieva, D.; Jóvári, P.; Hannon, A.C. Structure of rare-earth phosphate glasses by X-ray and neutron diffraction. J. Non-Cryst. Solids 2005, 351, 3179–3190. [Google Scholar] [CrossRef]
- Bachvarova-Nedelcheva, A.; Iordanova, R.; Ganev, S.; Dimitriev, Y. Glass formation and structural studies of glasses in the TeO2–ZnO–Bi2O3–Nb2O5 system. J. Non-Cryst. Solids 2019, 503, 224–231. [Google Scholar] [CrossRef]
- Gajek, M.; Leśniak, M.; Sitarz, M.; Stodolak-Zych, E.; Rapacz-Kmita, A. The crystallization and structure features of glass within the K2O–MgO–CaO–Al2O3–SiO2-(BaO) system. J. Mol. Struct. 2020, 1220, 128747. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, B.; Gong, Y.; Ren, Y.; Huo, M.; Wang, Y. Concentration effect of Yb3+ ions on the spectroscopic properties of high-concentration Er3+/Yb3+ co-doped phosphate glasses. J. Mol. Struct. 2020, 1216, 128322. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, H.; Zou, H.; Sun, Z.; Xu, Y.; Wang, C.; Xie, X.; Yi, J.; Zhao, F. Spectroscopic and Surface Crystallization Characterizations of Yttrium-Doped Phosphate Glasses. Crystals 2022, 12, 109. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010109
Li H, Zou H, Sun Z, Xu Y, Wang C, Xie X, Yi J, Zhao F. Spectroscopic and Surface Crystallization Characterizations of Yttrium-Doped Phosphate Glasses. Crystals. 2022; 12(1):109. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010109
Chicago/Turabian StyleLi, Haijian, Hao Zou, Zhihua Sun, Yi Xu, Changjian Wang, Xiao Xie, Jianhua Yi, and Fengqi Zhao. 2022. "Spectroscopic and Surface Crystallization Characterizations of Yttrium-Doped Phosphate Glasses" Crystals 12, no. 1: 109. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010109