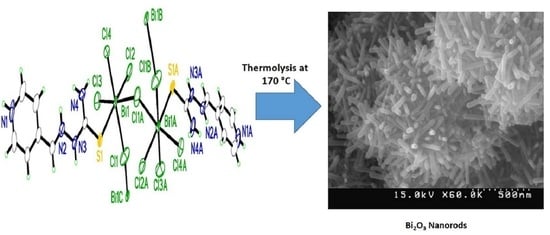
The Synthesis and Characterization of a Novel One-Dimensional Bismuth (III) Coordination Polymer as a Precursor for the Production of Bismuth (III) Oxide Nanorods
Abstract
:1. Introduction
2. Experiment
2.1. Materials
2.2. Isolated Single Crystal of [Bi(HQ)(Cl)4]n Synthesis
2.3. Synthesis of the Compound [Bi(HQ)(Cl)4]n in Bulk Form
2.4. Synthesis of the [Bi(HQ)(Cl)4]n Nanostructure
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Engel, E.R.; Scott, J.L. Advances in the green chemistry of coordination polymer materials. Green Chem. 2020, 22, 3693–3715. [Google Scholar] [CrossRef]
- Liu, J.Q.; Luo, Z.D.; Ying, P.; Singh, A.K.; Trivedi, M.; Kumar, A. Recent developments in luminescent coordination polymers: Designing strategies, sensing application and theoretical evidences. Coord. Chem. Rev. 2020, 406, 213145. [Google Scholar] [CrossRef]
- Farrusseng, D.; Aguado, S.; Pinel, C. Minireviews MOFs in Catalysis Metal—Organic Frameworks: Opportunities for Catalysis. Angew. Chem. Int. Ed. 2009, 48, 7502–7513. [Google Scholar] [CrossRef] [PubMed]
- Kurmoo, M. Magnetic metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1353. [Google Scholar] [CrossRef] [PubMed]
- Allendorf, M.D.; Bauer, C.A.; Bhakta, R.K.; Houk, R.J.T. Luminescent metal–organic frameworks. Chem. Soc. Rev. 2009, 38, 1330. [Google Scholar] [CrossRef]
- Horcajada, P.; Gref, R.; Baati, T.; Allan, P.K.; Maurin, G.; Couvreur, P.; Férey, G.; Morris, R.E.; Serre, C. [LER ESSE DE NOVO] Metal–Organic Frameworks in Biomedicine. Chem. Rev. 2012, 112, 1232–1268. [Google Scholar] [CrossRef] [PubMed]
- Bastani, D.; Esmaeili, N.; Asadollahi, M. Polymeric mixed matrix membranes containing zeolites as a filler for gas separation applications: A review. J. Ind. Eng. Chem. 2013, 19, 375–393. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhao, Y.; Gong, Q.; Li, Z.; Li, J. MOFs for CO2 capture and separation from flue gas mixtures: The effect of multifunctional sites on their adsorption capacity and selectivity. Chem. Commun. 2013, 49, 653–661. [Google Scholar] [CrossRef]
- Gu, J.Z.; Wen, M.; Liang, X.X.; Shi, Z.F.; Kirillova, M.V.; Kirillov, A.M. Multifunctional aromatic carboxylic acids as versatile building blocks for hydrothermal design of coordination polymers. Crystals 2018, 8, 83. [Google Scholar] [CrossRef] [Green Version]
- Song, B.H.; Ding, X.; Li, C.; An, G.F. Synthesis, crystal structures, and anti-liver cancer activity studies on three similar coordination polymers. Crystals 2018, 8, 207. [Google Scholar] [CrossRef] [Green Version]
- Dadashi, J.; Hanifehpour, Y.; Mirtamizdoust, B.; Abdolmaleki, M.; Jegarkandi, E.M.; Rezaei, M.; Joo, S.W. Ultrasound-Assisted Synthesis and DFT Calculations of the Novel 1D Pb (II) Coordination Polymer with Thiosemicarbazone Derivative Ligand and Its Use for Preparation of PbO Clusters. Crystals 2021, 11, 682. [Google Scholar] [CrossRef]
- Sorg, J.R.; Wehner, T.; Matthes, P.R.; Sure, R.; Grimme, S.; Heine, J.; Buschbaum, K.M. Bismuth as Versatile Cation for Luminescence in Coordination Polymers from BiX3/4,4-bipy: Understanding of Photophysics by Quantum Chemical Calculations and Structural Parallels to Lanthanides. Dalton Trans. 2018, 47, 7669–7681. [Google Scholar] [CrossRef]
- Andleeb, S.; Din, I. Recent progress in designing the synthetic strategies for bismuth based complexes. J. Organomet. Chem. 2019, 898, 120871. [Google Scholar] [CrossRef]
- Wibowo, A.C.; Smith, M.D.; Yeon, J.; Halasyamani, P.S.; Loye, H.C. Novel 3D bismuth-based coordination polymers: Synthesis, structure, and second harmonic generation properties. J. Solid State Chem. 2012, 195, 94–100. [Google Scholar] [CrossRef]
- Roodsari, M.S.; Shaabani, B.; Mirtamizdoust, B.; Dusek, M.; Fejfarova, K. Sonochemical Synthesis of Bismuth (III) Nano Coordination Compound and Direct Synthesis of Bi2O3 Nanoparticles from a Bismuth (III) Nano Coordination Compound Precursor. Inorg. Organomet. Polym. 2015, 25, 1226–1232. [Google Scholar] [CrossRef]
- Kowalik, M.; Masternak, J.; Łakomska, I.; Kazimierczuk, K.; Zawilak-Pawlik, A.; Szczepanowski, P.; Khavryuchenko, O.V.; Barszcz, B. Structural Insights into New Bi(III) Coordination Polymers with Pyridine-2,3-Dicarboxylic Acid: Photoluminescence Properties and Anti-Helicobacter pylori Activity. Int. J. Mol. Sci. 2020, 21, 8696. [Google Scholar] [CrossRef] [PubMed]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem. 2016, 29, 104–128. [Google Scholar] [CrossRef] [PubMed]
- Mason, T.J. Ultrasound in synthetic organic chemistry. Chem. Soc. Rev. 1997, 26, 443–451. [Google Scholar] [CrossRef]
- Suslick, K.S.; Price, G.J. Applications of ultrasound to materials chemistry. Ann. Rev. Mater. Sci. 1999, 29, 295–326. [Google Scholar] [CrossRef] [Green Version]
- Hanifehpour, Y.; Dadashi, J.; Mirtamizdoust, B. Ultrasound-Assisted Synthesis and Crystal Structure of Novel 2D Cd (II) Metal–Organic Coordination Polymer with Nitrite End Stop Ligand as a Precursor for Preparation of CdO Nanoparticles. Crystals 2021, 11, 197. [Google Scholar] [CrossRef]
- Mirtamizdoust, B.; Hanifehpour, Y.; Behzadfar, E.; Roodsari, M.S.; Jung, J.H.; Joo, S.W. A novel nano-structured three-dimensional supramolecular metal-organic framework for cadmium (II): A new precursor for producing nano cadmium oxide. J. Mol. Struct. 2020, 1201, 127191. [Google Scholar] [CrossRef]
- Hanifehpour, Y.; Mirtamizdoust, B.; Hatami, M.; Khomami, B.; Joo, S.W. Synthesis and structural characterization of new bismuth (III) nano coordination polymer: A precursor to produce pure phase nano-sized bismuth (III) oxide. J. Mol. Struct. 2015, 1091, 43–48. [Google Scholar] [CrossRef]
- Mercury 2.4: Copyright Cambridge Crystallographic Data Centre; Oxford Diffraction Limited: Cambridge, UK, 2001–2010.
- Oxford Diffraction. CrysAlis RED and CrysAlis CCD Software (Ver. 1.171.32.5); Oxford Diffraction Ltd.: Oxfordshire, UK, 2004. [Google Scholar]
- Mahmoud, W.E.; Al-Ghamdi, A.A.; Al-Agel, F. Synthesis and optical properties of poly (vinyl acetate)/bismuth oxide nanorods. Polym. Adv. Technol. 2011, 22, 2055–2061. [Google Scholar] [CrossRef]
- Solanki, P.R.; Singh, J.; Rupavali, B.; Tiwari, S.; Malhotra, B.D. Bismuth oxide nanorods based immunosensor for mycotoxin detection. Mater. Sci. Eng. C 2017, 70, 564–571. [Google Scholar] [CrossRef] [PubMed]
- Soltanzadeh, N.; Morsali, A. Sonochemical synthesis of a new nano-structures bismuth (III) supramolecular compound: New precursor for the preparation of bismuth (III) oxide nano-rods and bismuth (III) iodide nano-wires. Ultrason. Sonochem. 2010, 17, 139–144. [Google Scholar] [CrossRef]
- Luo, H.; Yang, R.G.; Chen, Z.H.; Zhong, G.Q. Three bismuth (III) complexes constructed by N-containing heterocyclic carboxylic acids: Synthesis, crystal structure and photocatalytic activity. J. Solid State Chem. 2021, 300, 122256. [Google Scholar] [CrossRef]
- Belghoul, B.; Welterlich, I.; Maier, A.; Toutianoush, A.; Rabindranath, A.R.; Tieke, B. Supramolecular sequential assembly of polymer thin films based on dimeric, dendrimeric, and polymeric schiff-base ligands and metal ions. Langmuir 2007, 23, 5062–5069. [Google Scholar] [CrossRef]
- Fan, H.T.; Pan, S.S.; Teng, X.M.; Ye, C.; Li, G.H.; Zhang, L.D. δ-Bi2O3 thin films prepared by reactive sputtering: Fabrication and characterization. Thin Solid Films 2006, 513, 142–147. [Google Scholar] [CrossRef]
- Shinde, P.V.; Ghule, B.G.; Shaikh, S.F.; Shinde, N.M.; Sangale, S.S.; Jadhav, V.V.; Yoon, S.-Y.; Kim, K.H.; Mane, R.S. Microwave-assisted hierarchical bismuth oxide worm-like nanostructured films as room-temperature hydrogen gas sensors. J. Alloy. Compd. 2019, 802, 244–251. [Google Scholar] [CrossRef]
- Zingg, D.S.; Hercules, D.M. Electron spectroscopy for chemical analysis studies of lead sulfide oxidation. J. Phys. Chem. 1978, 82, 1992–1995. [Google Scholar] [CrossRef]















| Chemical Formula | C7H9BiCl4N4S |
|---|---|
| Mr | 532.02 |
| Crystal system, space group | Monoclinic, P21/c |
| Temperature (K) | 298(2) |
| a, b, c (Å) | 11.3205 (4), 7.4736 (3), 17.8244 (6) |
| β(°) | 97.9430(10)° |
| V(Å3) | 1493.56(9) |
| Z | 4 |
| Crystal size (mm3) | 0.200 × 0.100 × 0.030 |
| Theta range for data collection | 3.943 to 72.090° |
| Max. and min. transmission | 0.7535, 0.2404 |
| Rint | 0.0574 |
| Absorption coefficient mm−1 | 30.948 |
| R,wR2 [I > 2σ(I)] | 0.0838, 0.2181 |
| R, wR2 (all data) | 0.0856, 0.2247 |
| No. of reflections | 8804 |
| No. of parameters | 155 |
| No. of restraints | 0 |
| Absorption correction | Multi-scan (Bruker SADABS) |
| Δ›max, Δ›min (e Å−3) | 5.003, −4.488 |
| Goodness-of-fit on F2 | 1.060 |
| Bi(1)-Cl(3) | 2.542(3) | Cl(3)-Bi(1)-Cl(4) | 89.73(11) |
| Bi(1)-Cl(4) | 2.620(2) | Cl(3)-Bi(1)-Cl(2) | 88.27(14) |
| Bi(1)-Cl(2) | 2.714(4) | Cl(4)-Bi(1)-Cl(2) | 91.25(12) |
| Bi(1)-Cl(1) | 2.769(4) | Cl(3)-Bi(1)-Cl(1) | 97.02(14) |
| Bi(1)-S(1) | 2.841(3) | Cl(4)-Bi(1)-Cl(1) | 170.90(12) |
| Bi(1)-Cl(1)#1 | 2.861(3) | Cl(2)-Bi(1)-Cl(1) | 95.03(16) |
| N(1)-H(1A) | 0.86 | Cl(3)-Bi(1)-S(1) | 89.60(12) |
| Cl(1)-Bi(1)-S(1) | 80.60(15) | Cl(4)-Bi(1)-S(1) | 93.41(9) |
| Cl(3)-Bi(1)-Cl(1)#1 | 173.81(14) | Cl(2)-Bi(1)-S(1) | 174.87(13) |
| Cl(4)-Bi(1)-Cl(1)#1 | 85.71(12) | Cl(2)-Bi(1)-Cl(1)#1 | 87.64(17) |
| S(1)-Bi(1)-Cl(1)#1 | 94.85(14) | Cl(1)-Bi(1)-Cl(1)#1 | 87.97(5) |
| Bi(1)-Cl(1)-Bi(1)#2 | 147.3(2) |
| D-H...A | d(D-H) | d(H...A) | d(D...A) | <(DHA) |
|---|---|---|---|---|
| N1—H1A···Cl2 i | 0.86 | 2.25 | 3.103 (5) | 170.2 |
| N3—H3A···Cl2 ii | 0.86 | 2.36 | 3.205 (5) | 169.5 |
| N4—H4A···Cl3 iii | 0.86 | 2.61 | 3.247 (5) | 131.9 |
| N4—H4A···Cl4 iv | 0.86 | 2.95 | 2.452 (5) | 119.1 |
| N4—H4B···Cl4 | 0.86 | 2.34 | 3.195 (5) | 170.0 |
| C5—H5A···Cl3 v | 0.93 | 2.91 | 3.661 (5) | 138.9 |
| C6—H6A···Cl4 ii | 0.93 | 2.89 | 3.720 (5) | 149.8 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hanifehpour, Y.; Mirtamizdoust, B.; Dadashi, J.; Wang, R.; Rezaei, M.; Abdolmaleki, M.; Joo, S.W. The Synthesis and Characterization of a Novel One-Dimensional Bismuth (III) Coordination Polymer as a Precursor for the Production of Bismuth (III) Oxide Nanorods. Crystals 2022, 12, 113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010113
Hanifehpour Y, Mirtamizdoust B, Dadashi J, Wang R, Rezaei M, Abdolmaleki M, Joo SW. The Synthesis and Characterization of a Novel One-Dimensional Bismuth (III) Coordination Polymer as a Precursor for the Production of Bismuth (III) Oxide Nanorods. Crystals. 2022; 12(1):113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010113
Chicago/Turabian StyleHanifehpour, Younes, Babak Mirtamizdoust, Jaber Dadashi, Ruiyao Wang, Mahboube Rezaei, Mehdi Abdolmaleki, and Sang Woo Joo. 2022. "The Synthesis and Characterization of a Novel One-Dimensional Bismuth (III) Coordination Polymer as a Precursor for the Production of Bismuth (III) Oxide Nanorods" Crystals 12, no. 1: 113. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010113