Synthesis of Spinel-Hydroxyapatite Composite Utilizing Bovine Bone and Beverage Can
Abstract
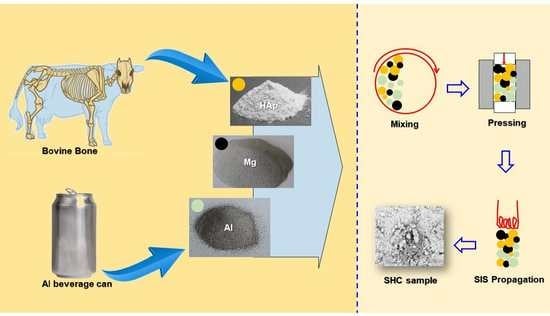
:1. Introduction
2. Materials and Methods
2.1. Bovine Bones Treatment
2.2. Aluminum Cans Treatment
2.3. Self-Propagating Intermediate-Temperature Synthesis (SIS)
3. Results and Discussions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, S.; Wang, J.; Zou, L.; Jin, L.; Wang, Z.; Li, Y. A Three-Dimensional Hydroxyapatite/ Polyacrylonitrile Composite Scaffold Designed for Bone Tissue Engineering. RSC Adv. 2018, 8, 1730–1736. [Google Scholar] [CrossRef] [Green Version]
- Sari, Y.W.; Rudianto, R.P.; Nuzulia, N.A.; Sukaryo, S.G. Injectable Bone Substitute Synthesized from Mangrove Snail Shell. J. Med. Phys. Biophys. 2017, 4, 115–121. [Google Scholar]
- Odusote, J.K.; Danyuo, Y.; Baruwa, A.D.; Azeez, A.A. Synthesis and Characterization of Hydroxyapatite from Bovine Bone for Production of Dental Implants. J. Appl. Biomater. Funct. Mater. 2019, 17, 2280800019836829. [Google Scholar] [CrossRef]
- Pon-On, W.; Suntornsaratoon, P.; Charoenphandhu, N.; Thongbunchoo, J.; Krishnamra, N.; Tang, I.M. Hydroxyapatite from Fish Scale for Potential Use as Bone Scaffold or Regenerative Material. Mater. Sci. Eng. C 2016, 62, 183–189. [Google Scholar] [CrossRef]
- Vignesh Raj, S.; Rajkumar, M.; Meenakshi Sundaram, N.; Kandaswamy, A. Synthesis and Characterization of Hydroxyapatite/Alumina Ceramic Nanocomposites for Biomedical Applications. Bull. Mater. Sci. 2018, 41, 93. [Google Scholar] [CrossRef] [Green Version]
- Budiatin, A.S.; Gani, M.A.; Samirah; Ardianto, C.; Raharjanti, A.M.; Septiani, I.; Putri, N.P.K.P.; Khotib, J. Bovine Hydroxyapatite-Based Bone Scaffold with Gentamicin Accelerates Vascularization and Remodeling of Bone Defect. Int. J. Biomater. 2021, 2021, 5560891. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Hsiao, P.; Chai, H. Hydroxyapatite Extracted from Fish Scale: Effects on MG63 Osteoblast-like Cells. Ceram. Int. 2011, 37, 1825–1831. [Google Scholar] [CrossRef]
- Kumar, G.S.; Sathish, L.; Govindan, R.; Girija, E.K. Utilization of Snail Shells to Synthesise Hydroxyapatite Nanorods for Orthopedic Applications. RSC Adv. 2015, 5, 39544–39548. [Google Scholar] [CrossRef]
- Abdulrahman, I.; Tijani, H.I.; Mohammed, B.A.; Saidu, H.; Yusuf, H.; Ndejiko Jibrin, M.; Mohammed, S. From Garbage to Biomaterials: An Overview on Egg Shell Based Hydroxyapatite. J. Mater. 2014, 2014, 802467. [Google Scholar] [CrossRef]
- Kattimani, V.; Lingamaneni, K.P.; Chakravarthi, P.S.; Sampath Kumar, T.S.; Siddharthan, A. Eggshell-Derived Hydroxyapatite: A New Era in Bone Regeneration. J. Craniofac. Surg. 2016, 27, 112–117. [Google Scholar] [CrossRef] [PubMed]
- Radha, R.; Sreekanth, D. Mechanical and Corrosion Behaviour of Hydroxyapatite Reinforced Mg-Sn Alloy Composite by Squeeze Casting for Biomedical Applications. J. Magnes. Alloy. 2020, 8, 452–460. [Google Scholar] [CrossRef]
- Witte, F.; Feyerabend, F.; Maier, P.; Fischer, J.; Störmer, M.; Blawert, C.; Dietzel, W.; Hort, N. Biodegradable Magnesium-Hydroxyapatite Metal Matrix Composites. Biomaterials 2007, 28, 2163–2174. [Google Scholar] [CrossRef] [Green Version]
- Lim, P.N.; Lam, R.N.; Zheng, Y.F.; Thian, E.S. Magnesium-Calcium/Hydroxyapatite (Mg-Ca/HA) Composites with Enhanced Bone Differentiation Properties for Orthopedic Applications. Mater. Lett. 2016, 172, 193–197. [Google Scholar] [CrossRef]
- Del Campo, R.; Savoini, B.; Muñoz, A.; Monge, M.A.; Garcés, G. Mechanical Properties and Corrosion Behavior of Mg-HAP Composites. J. Mech. Behav. Biomed. Mater. 2014, 39, 238–246. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Fartash, B.; Hermansson, L. Hydroxyapatite-Alumina Composites and Bone-Bonding. Biomaterials 1995, 16, 417–422. [Google Scholar] [CrossRef]
- Yoon, H.J.; Yoon, J.H.; Park, S.H.; Lee, M.H.; Han, J.S.; Kim, D.J. The Role of MgAl2O4 Powder Packing on Phase Stability of Hydroxyapatite during Sintering. J. Am. Ceram. Soc. 2015, 98, 1787–1793. [Google Scholar] [CrossRef]
- Sreekanth, D.; Rameshbabu, N. Development and Characterization of MgO/Hydroxyapatite Composite Coating on AZ31 Magnesium Alloy by Plasma Electrolytic Oxidation Coupled with Electrophoretic Deposition. Mater. Lett. 2012, 68, 439–442. [Google Scholar] [CrossRef]
- Wei, G.; Ma, P.X. Structure and Properties of Nano-Hydroxyapatite/Polymer Composite Scaffolds for Bone Tissue Engineering. Biomaterials 2004, 25, 4749–4757. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Apanasevich, V.I.; Plekhova, N.G.; Buravlev, I.Y.; Zinoviev, S.V.; Mayorov, V.Y.; Fedorets, A.N.; Merkulov, E.B.; Shlyk, D.K.; et al. Synthetic Nanostructured Wollastonite: Composition, Structure and “in Vitro” Biocompatibility Investigation. Ceram. Int. 2021, 47, 22487–22496. [Google Scholar] [CrossRef]
- Papynov, E.K.; Shichalin, O.O.; Buravlev, I.Y.; Portnyagin, A.S.; Belov, A.A.; Maiorov, V.Y.; Skurikhina, Y.E.; Merkulov, E.B.; Glavinskaya, V.O.; Nomerovskii, A.D.; et al. Reactive Spark Plasma Synthesis of Porous Bioceramic Wollastonite. Russ. J. Inorg. Chem. 2020, 65, 263–270. [Google Scholar] [CrossRef]
- Risonarta, V.Y.; Anggono, J.; Suhendra, Y.M.; Nugrowibowo, S.; Jani, Y. Strategy to Improve Recycling Yield of Aluminium Cans. E3S Web Conf. 2019, 130, 01033. [Google Scholar] [CrossRef]
- Begum, S. Recycling of Aluminum from Aluminum Cans. J. Chem. Soc. Pakistan 2013, 35, 1490–1493. [Google Scholar]
- Akrom, M.; Marwoto, P. Sugianto Pembuatan Mmc Berbasis Teknologi Metalurgi Serbuk Dengan Bahan Baku Aluminium Dari Limbah Kaleng Minuman Dan Aditif Abu Sekam Padi. J. Pendidik. Fis. Indones. 2010, 6, 14–19. [Google Scholar]
- Slotwinski, J.A.; Garboczi, E.J.; Hebenstreit, K.M. Porosity Measurements and Analysis for Metal Additive Manufacturing Process Control. J. Res. Natl. Inst. Stand. Technol. 2014, 119, 494–528. [Google Scholar] [CrossRef] [PubMed]
- Converse, G.L.; Conrad, T.L.; Merrill, C.H.; Roeder, R.K. Hydroxyapatite Whisker-Reinforced Polyetherketoneketone Bone Ingrowth Scaffolds. Acta Biomater. 2010, 6, 856–863. [Google Scholar] [CrossRef] [PubMed]
- Poovazhagan, L.; Rajkumar, K.; Saravanamuthukumar, P.; Javed Syed Ibrahim, S.; Santhosh, S. Effect of Magnesium Addition on Processing the Al-0.8 Mg-0.7 Si/SiCp Metal Matrix Composites. Appl. Mech. Mater. 2015, 787, 553–557. [Google Scholar] [CrossRef]
- Sangghaleh, A.; Halali, M. Effect of Magnesium Addition on the Wetting of Alumina by Aluminium. Appl. Surf. Sci. 2009, 255, 8202–8206. [Google Scholar] [CrossRef]
- Hasanah, I.U.; Fadhillah, M.F.; Putra, Y.V. Fabrication and Characterization of Aluminum Matrix Composite (AMCs) Reinforced Graphite by Stir Casting Method for Automotive Application. Mater. Sci. Forum 2020, 988, 17–22. [Google Scholar]
- Dahi, E. Optimisation of Bone Char Production Using the Standard Defluoridation Capacity Procedure. Fluoride 2015, 48, 29–36. [Google Scholar]
- Fadhilah, N.; Irhamni, I.; Jalil, Z. Synthesis of Natural Hydroxyapatite from Aceh’s Bovine Bone. J. Aceh Phys. Soc. 2016, 5, 19–21. [Google Scholar]
- Londoño-Restrepo, S.M.; Herrera-Lara, M.; Bernal-Alvarez, L.R.; Rivera-Muñoz, E.M.; Rodriguez-García, M.E. In-Situ XRD Study of the Crystal Size Transition of Hydroxyapatite from Swine Bone. Ceram. Int. 2020, 46, 24454–24461. [Google Scholar] [CrossRef]
- Singh, T.P.; Singh, H.; Singh, H. Characterization of Thermal Sprayed Hydroxyapatite Coatings on Some Biomedical Implant Materials. J. Appl. Biomater. Funct. Mater. 2014, 12, 48–56. [Google Scholar] [CrossRef]
- Mahabole, M.; Bahir, M.; Khairnar, R. Mn Blended Hydroxyapatite Nanoceramic: Bioactivity, Dielectric and Luminescence Studies. J. Biomim. Biomater. Tissue Eng. 2013, 18, 43–59. [Google Scholar] [CrossRef]
- Paluszkiewicz, C.; Ślósarczyk, A.; Pijocha, D.; Sitarz, M.; Bućko, M.; Zima, A.; Chróścicka, A.; Lewandowska-Szumieł, M. Synthesis, Structural Properties and Thermal Stability of Mn-Doped Hydroxyapatite. J. Mol. Struct. 2010, 976, 301–309. [Google Scholar] [CrossRef]
- Li, Y.; Widodo, J.; Lim, S.; Ooi, C.P. Synthesis and Cytocompatibility of Manganese (II) and Iron (III) Substituted Hydroxyapatite Nanoparticles. J. Mater. Sci. 2012, 47, 754–763. [Google Scholar] [CrossRef]
- Panseri, S.; Cunha, C.; D’Alessandro, T.; Sandri, M.; Giavaresi, G.; Marcacci, M.; Hung, C.T.; Tampieri, A. Intrinsically Superparamagnetic Fe-Hydroxyapatite Nanoparticles Positively Influence Osteoblast-like Cell Behaviour. J. Nanobiotechnol. 2012, 10, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaygili, O.; Dorozhkin, S.V.; Ates, T.; Al-Ghamdi, A.A.; Yakuphanoglu, F. Dielectric Properties of Fe Doped Hydroxyapatite Prepared by Sol-Gel Method. Ceram. Int. 2014, 40, 9395–9402. [Google Scholar] [CrossRef]
- Bianco, A.; Cacciotti, I.; Lombardi, M.; Montanaro, L. Si-Substituted Hydroxyapatite Nanopowders: Synthesis, Thermal Stability and Sinterability. Mater. Res. Bull. 2009, 44, 345–354. [Google Scholar] [CrossRef]
- Hadidi, M.; Bigham, A.; Saebnoori, E.; Hassanzadeh-Tabrizi, S.A.; Rahmati, S.; Alizadeh, Z.M.; Nasirian, V.; Rafienia, M. Electrophoretic-Deposited Hydroxyapatite-Copper Nanocomposite as an Antibacterial Coating for Biomedical Applications. Surf. Coat. Technol. 2017, 321, 171–179. [Google Scholar] [CrossRef]
- Oh, S.-T.; Sekino, T.; Niihara, K. Effect of Particle Size Distribution and Mixing Homogeneity on Microstructure and Strength of Alumina/Copper Composites. Nanostruct. Mater. 1998, 10, 327–332. [Google Scholar] [CrossRef]
- Halverson, D.C.; Pyzik, A.J.; Aksay, I.A.; Snowden, W.E. Processing of Boron Carbide-Aluminum Composites. J. Am. Ceram. Soc. 1989, 72, 775–780. [Google Scholar] [CrossRef] [Green Version]
- Del Gutiérrez-Salazar, M.P.; Reyes-Gasga, J. Microhardness and Chemical Composition of Human Tooth. Mater. Res. 2003, 6, 367–373. [Google Scholar] [CrossRef] [Green Version]
- Craıg, R.G.; Peyton, F.A. The Micro-Hardness of Enamel and Dentin. J. Dent. Res. 1958, 37, 661–668. [Google Scholar] [CrossRef]
- Pramanik, S.; Agarwal, A.K.; Rai, K.N. Development of High Strength Hydroxyapatite for Hard Tissue Replacement. Trends Biomater. Artif. Organs 2005, 19, 45–49. [Google Scholar]
- Keaveny, T.M.; Morgan, E.F.; Yeh, O.C. Bone Mechanics. In Standard Handbook of Biomedical Engineering and Design; Kutz, M., Ed.; The McGraw-Hill Companies, Inc.: New York, NY, USA, 2004; pp. 8.1–8.23. ISBN 9780071356374. [Google Scholar]
- Al-Sanabani, F.A.; Madfa, A.A.; Al-Qudaini, N. Alumina Ceramic for Dental Applications: A Review Article. Am. J. Mater. Res. 2014, 1, 26–34. [Google Scholar]
- Piconi, C.; Maccauro, G.; Muratori, F.; Brach Del Prever, E. Alumina and Zirconia Ceramics in Joint Replacements. J. Appl. Biomater. Biomech. 2003, 1, 19–32. [Google Scholar]
- Latief, F.H.; Chafidz, A.; Junaedi, H.; Alfozan, A.; Khan, R. Effect of Alumina Contents on the Physicomechanical Properties of Alumina (Al2O3) Reinforced Polyester Composites. Adv. Polym. Technol. 2019, 2019, 5173537. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, K.; Zhu, K.; Fujino, T.; Onda, A. Preparation of Hydroxyapatite Ceramics by Hydrothermal Hot-Pressing Technique. Key Eng. Mater. 2006, 309–311, 57–60. [Google Scholar] [CrossRef]







| Sample | HAp-A | HAp-B | HAp-C | HAp-D | Reference * |
|---|---|---|---|---|---|
| a (Å) | 9.387 | 9.425 | 9.423 | 9.426 | 9.421 |
| c (Å) | 6.885 | 6.883 | 6.883 | 6.882 | 6.893 |
| Elements | O | P | Ca | Total |
|---|---|---|---|---|
| at. % | 69.05 | 12.54 | 18.41 | 100.00 |
| Elements | Percentage (%) | Elements | Percentage (%) |
|---|---|---|---|
| Al | 91.90 ± 1.50 | Ni | 0.06 ± 0.02 |
| Mn | 3.20 ± 0.40 | Zr | 0.03 ± 0.03 |
| Si | 2.80 ± 0.80 | Ti | 0.02 ± 0.09 |
| Fe | 1.39 ± 0.19 | Zn | 0.01 ± 0.01 |
| Cu | 0.62 ± 0.12 |
| Elements | at. % | |
|---|---|---|
| <90 µm | >150 µm | |
| O | 66.75 | 66.60 |
| Mg | 2.47 | 2.12 |
| Al | 2.46 | 4.22 |
| P | 11.45 | 10.20 |
| Ca | 16.87 | 14.86 |
| Total | 100.00 | 100.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pramono, A.; Timuda, G.E.; Rifai, G.P.A.; Khaerudini, D.S. Synthesis of Spinel-Hydroxyapatite Composite Utilizing Bovine Bone and Beverage Can. Crystals 2022, 12, 96. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010096
Pramono A, Timuda GE, Rifai GPA, Khaerudini DS. Synthesis of Spinel-Hydroxyapatite Composite Utilizing Bovine Bone and Beverage Can. Crystals. 2022; 12(1):96. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010096
Chicago/Turabian StylePramono, Agus, Gerald Ensang Timuda, Ganang Pramudya Ahmad Rifai, and Deni Shidqi Khaerudini. 2022. "Synthesis of Spinel-Hydroxyapatite Composite Utilizing Bovine Bone and Beverage Can" Crystals 12, no. 1: 96. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst12010096