1. Introduction
Hydrogen is a well known impurity in semiconductors with prominent impact on electrical, structural and optical properties. Intentional hydrogen incorporation (generally by plasma or implantation) allows tuning the bandgap and consequently improves solar light absorption caused by both reduction of ions and hydrogen doping [
1]. Behaviour of hydrogen in semiconductors is far from being straightforward. Despite a multitude of possible states (H
, H
, H
and H
) hydrogen can take, there is a mere handful of information available, even for most used compounds. Depending on a semiconductor, hydrogen behaves differently. In wide-band-metal-oxides, hydrogen tends to bound to oxygen and can mainly act as an amphoteric impurity or as a donor [
2]. The latter is the case for TiO
. In stable rutile, oxygen vacancies are also considered contributing to the system. However, when there is an excess of electrons they tend to be localised at the Ti site, giving rise to a polaron formation. Moreover, a polaron remains favourable even when additional electrons are induced via a dopant [
3,
4]. In the 70s, an improvement of
n-type conductivity was spotted after hydrogenation in TiO
. Interstitial hydrogen was suggested to form a single dative bond to an oxygen atom, where hydrogen was located in the open
c channel of the crystal [
5].
More recent studies with IR absorption spectroscopy have shown that the hydrogen nature in metal oxides is far more complicated. It has been shown that hydrogen could feature several forms stable against low temperature annealing, and furthermore, if it is stabilised by a defect nearby can remain in the lattice up to 1150 K [
6,
7,
8,
9]. Stability of these complexes against other impacts (
ion implantation, stress) raises questions. Under IR and electron irradiation, diffusivity of hydrogen has significantly improved and is anisotropic, with the activation barrier remarkable lower in the
c-direction [
10,
11].
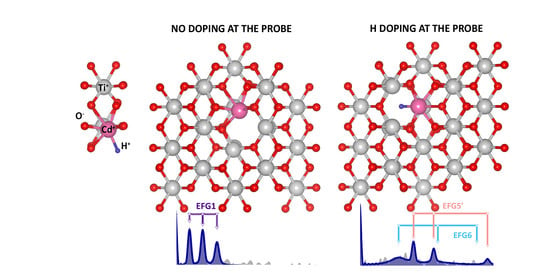
One of the subtle methods to probe structure, electronic properties and stability of impurity complexes in solids at the atomic scale is perturbed angular correlation spectroscopy. PAC is sensitive to the local electric field gradients (EFG) present in the lattice near the probe atom. Not only the method provides the information on structure and stability, it also allows spotting dynamical processes (correlation times in the order of
s or even ns), which is of big help for fast diffusion phenomena and fluctuation of charge states. An electric field gradient is caused by a charge distribution, which deviates from an ideal spherically symmetric arrangement around the implanted probe. The EFG is usually described by the quadrupole coupling constant
V
(here
Q is nuclear quadrupole moment and V
is the largest component of the EFG tensor) and asymmetry parameter,
. The latter can only take values between 0 and 1, and measures how far from axial symmetry a charge distribution deviates:
= (V
− V
)/V
. In the current study, we are going to use
=
* (3
/10) as the spin of the Cd 245 keV probe state is
I= 5/2
. When
≃ 0 due to the high symmetry of the lattice and/or complex defects
=
is the smallest of the three observable frequencies characterising each EFGs, as
for
I = 5/2. More details about the method can be found elsewhere [
12]. The only significant bottleneck of the method is few suitable isotopes. However, ISOLDE/CERN facility provides the user with a great variety of suitable short-lived isotopes [
13].
The method has been previously applied for studying acceptor complexes, passivation, influence of pressure and annealing on hydrogen bonds dissociation and impact of hydrogen on the electric field gradient in semiconductors (Si, GaP, InP, and InAs) [
14]. In those studies hydrogen was introduced in several ways, including hydrogen plasma or low energy implantation. In the case of Si, for instance, hydrogen formed pairs with the majority of impurity probes (up to 80%) and its incorporation in the lattice did not influence the axial asymmetry parameter,
, of the EFG, and only alterations of the main observable
frequencies evidenced the changes [
15]. Studies performed with another hyperfine interaction method -
Fe emission Mössbauer spectroscopy on hydrogen plasma treated anatase samples have shown that hydrogen complexes are likely to be featured in at least two main forms: interstitial (or weakly bounded with longer bonds) H
and hydrogen with a covalent bond to an oxygen atom H
. These complexes have different activation energy, and thus their thermal motion was triggered in two consequent steps: (1) H
moves through equivalent positions (2) H
, once activation energy is sufficient to break its bond [
16]. In the case of highly doped and V
o-abundant samples, both steps could overlap. Therefore, it would be of big interest to go through these steps with PAC spectroscopy, as it provides several advantages over Mössbauer spectroscopy, especially at elevated temperatures. However, within the present study we performed several PAC experiments with
Cd probes on TiO
anatase samples and obtained no perturbations, as it also happened in the case of
Ag/Cd isotope [
17]. The reason behind the flat response of the perturbation function in anatase could be attributed to the formation of different charge states of the daughter probe, thus leading to a tiny V
, and consequently causing no perturbation of the time spectrum. Correspondingly, as pristine rutile behaves similarly, as anatase does [
16,
18], here we aim to spot ongoing changes in the electronic structure of hydrogenated rutile and to follow the behaviour of various environments on the verge between the first and second dissociation stages (in the vicinity of 570-580 K) [
9,
16].
2. Materials and Methods
TiO thin films were prepared by radio frequency sputtering (LA 440S by VON ARDENNE) onto cm Si substrates, utilising a ceramic TiO target (99.9% FHR Anlagenbau, Germany). During the process, the sputtering power was kept at 210 W and the Ar flux was fixed at 80 sccm. The thickness of thin films was controlled with an ellipsometer (Sentech SE 801) and was in order of 500 nm. Prior to hydrogenation, the samples were annealed at 1073 K for 5 h with slow cooling, to ensure their full crystallisation into rutile. Thereafter, the samples were treated in a chamber for plasma-enhanced hydrogenation, where an inductively coupled plasma instrument (Plasma Lab 100 ICP-CVD, Oxford Instruments) was used. The H plasma treatment was performed for 30 min in a broad temperature range of the substrate specifically at: room temperature (RT), 423, 473, 573 and 663 K. The ICP power was held at 3000 W, the chamber pressure at 3.5 Pa, and the H flow rate at 50 sccm.
PAC experiments were carried out on a basis of 6-detector TDPAC spectrometer equipped with LaBr
detectors placed around a tubular furnace at
geometry with time resolution (800 ps) [
19]. Isotopes of
Cd/Cd were implanted at room temperature into samples with energy of 30 keV and fluence in the order of
11 at/cm
2 provided by the ISOLDE radioactive beam facility at CERN [
20]. Usually, each sample is measured once, with a repetition if statistical quality is not sufficient. Since, the hydrogenation was done prior to the implantation, the radiation-damage-recovery-annealings were performed during the measurements up to a certain extent. This decision was based on an earlier study of implantation damage in rutile, where approximately a significant amount of the total damage was recovered near 500 K [
21], with the majority of damage affected in the near surface regions.
The chosen
Cd isotope decays to
111Cd via a cascade of two consecutive gamma rays of 151–245 keV, without inducing electronic excitation of the probe atom. The intermediate state has a lifetime t
= 85 ns, while the parent state decays with t
= 48.5 min. SRIM calculations [
22]) showed that the depth of implantation should not exceed 30 nm (average 15 nm). Quadrupole moment used for determination of the EFG (V
) is 0.664(7)b [
23].
X-ray diffraction patterns were recorded with a SIEMENS/BRUKER D 5000 X-ray diffraction using Cu-K radiation at 40 kV and 40 mA, with a pristine sample being scanned from to with a step size of in Grazing incidence (GIXRD).
3. Results and Discussions
Figure 1 shows the observable R(t) spectra and their Fourier transforms, as measured at 581 K after implantation of
Cd, for pristine and several TiO
samples hydrogenated at different temperatures.
Table 1 summarises the fitting parameters of the main fractions of
Cd probe nuclei interacting with different EFG distributions. Description of the results is divided into sections to facilitate the discussion flow.
3.1. Pristine Rutile
Annealing at 581 K seems to be sufficient to recover most of the implantation damage of the pristine sample, where solely one fraction of a single triplet of frequencies is seen to be present in the corresponding R(t) Fourier spectrum. The corresponding R(t) function has a well-defined pattern, what is the characteristic of a single EFG/environment for the Cd atoms in TiO
, being only slightly damped as a function of time. The electric field gradient (EFG 1) resembles the values previously obtained on rutile [
24,
25]. Absence of significant remaining implantation damage is evidenced by several factors. Firstly, one points to the small damping of the R(t) function that reveals a low density of defects, with an essentially defect free local environment. Secondly, the main frequency measured at this temperature
= 96.52(3) Mrad·s
−1 matches closely with previously reported values on rutile with Cd isotope [
24,
26]
= 98.5(2) Mrad·s
−1.
The non-zero axially asymmetry parameter resembles results already measured at room temperature, although being slightly higher (
= 0.19(2)) than previously measured values on single crystals (
= 0.07(1)) [
24,
25]. Based on these results, one may presume that most of the atoms reside at the Ti substitutional site, in an essentially defects-free environment. However, the reason for the increased value can be induced by the nature of the thin films (lattice mismatch between TiO
and Si) and generally formation of non-ideal crystal structure, on contrary to single crystals [
24]. Furthermore, as the annealing and measurement were performed simultaneously in oxygen-lacking-atmosphere, it can facilitate formation of oxygen scarce regions, where certain deviations from stoichiometry could occur.
3.2. Hydrogenated at RT
After hydrogenation the spectra became remarkably complex and, without Fourier transform analysis, it would be challenging to distinguish amongst the variety of local environments/EFGs and their corresponding fractions. Upon hydrogenation treatment at RT, there is no evidence for EFG1. The spectrum was best fitted with the help of three EFG distributions, leading to a strongly damped R(t) function, which is evidence for Cd atoms in a variety of local environments, characterised by EFG2, 3 and 4. One may point, that the limit of solubility of deuterium in metal oxides is temperature dependent [
27]; consequently, hydrogenation treatment at RT is likely not efficient to introduce and promote hydrogen deeply and homogeneously into the lattice. Thus, a possible scenario, leading to the current PAC results in such sample, hints the existence of complex of hydrogen-related defects. These defects, upon interaction with the Cd probe, lead to a plethora of local environments, whilst Cd could still reside at or near the Ti sites. The actual matching of the
Cd atoms distribution regarding the distribution of hydrogen is unknown. Most likely, their relative distributions, of diffused hydrogen and implanted Cd are not homogeneously overlapping.
At the limit of the experimental resolving power—statistics-wise, the assigned EFG2 contributes with small 8.4(5)% of a relatively undamped perturbation function (i.e., the signature of a relatively regular defect configuration). EFG3 contributes with 28(5) and has close V and EFG parameters such as EFG2, but demonstrating a much wider distribution, i.e., revealing the increase of defects density nearby. EFG4, being the principal contribution to the perturbation function (64(7)%), lastly characterises a fairly broad EFG distribution with a different characteristic V and . EFG4 likely evidences severe disorder induced during hydrogenation, which could not recover fast enough to be observed during the first ∼1 h of measurements performed at 581 K, when most of the statistics contribute to the TDPAC measurement.
It would be difficult to say, without modelling of defects with theoretical calculations, what are the reasons of the observed EFGs. Regardless of that, although some preferential configurations of Cd related defects might prevail, these cannot be resolved due to the still high concentration of randomly distributed H atoms or H-related complex defects.
It is noteworthy that, the particular H-complex defect configurations formed during hydrogenation at RT, might be correlated with intrinsic defects in TiO created during that process. We suggest that the “likely” high density of poorly diffusing hydrogen atoms in the TiO lattice induces the generation of intrinsic defects. This situation leads to the formation of complex hydrogen-lattice defects that are stable enough to remain observable during the TDPAC measurements performed at 581 K. However, as soon as the sample is heated during hydrogenation most of such complex defects will not predominate, e.g., after hydrogenation at 423 K, as observed during the following TDPAC experiments. This fact suggests that temperature activated hydrogenation with enhanced H-diffusion reduces the number of H-complex defects as well as H-lattice correlated defects.
3.3. Hydrogenated at 423 K
Measurements performed on the sample hydrogenated at 423 K open a pathway for further discussions. The spectrum is analysed in terms of three broad EFG distributions, namely EFG1, EFG3 and EFG5. The corresponding R(t) function has a slightly clearer and more developed shape, though still shows evidence for the broad EFG distributions. Seemingly, the current hydrogenation temperature allowed to bring back a small 17(3)% fraction of EFG1 (designated here as EFG1), which is characterised with similar to EFG1 V and electric field gradient parameters.
This fraction demonstrates a relatively narrow EFG distribution. The next component, EFG3, may have the same nature as the one formed after hydrogenation at RT—EFG3. In comparison to EFG3, one may notice a growth of the principal contribution to the perturbation function from 28(5)% to 45(4)%, whilst V and the wide distribution remain almost the same, = 3.5(1)% and = 7(1)%.
Apparently, the complex defects change and lead to more axially asymmetric EFGs signatures, as seen through the growth of up to 0.83. EFG5, characterised with V = 18.91(4)21 V·m−2 and = 0.68(2), may be due to the further temperature development during hydrogenation of the defect configuration responsible for EFG4, with a similar wide EFG distribution. However, significant changes in the main frequency and axial asymmetry parameters, between EFG4 and EFG5 require that by no ways Cd is interacting with the same defect complex. At this point, one may argue that hydrogenation performed at 423 K is not entirely efficient to avoid the formation of still, e.g., H-lattice highly stable defects. Therefore, the current temperature step and its evaluation is not entirely justified, as it can be treated in several possible ways due to the local complexity and absence of the defined frequencies. Regardless, at this stage the material seems to be less damaged that further allowed a certain recuperation of implantation defects at relatively H-free zones for the implanted Cd probe atoms. Possibly, bestowed energy during hydrogenation, leading to higher dilution and less complex defects, allows hydrogen to interact more freely with the Cd acceptors in multiple ways, as might be seen in the case of samples hydrogenated at higher temperatures.
3.4. Hydrogenated at 473 K
Upon hydrogenation at 473 K the corresponding R(t) function became even more well-defined and solely two different EFGs are needed for analysis (EFG5
and EFG6). One can notice that EFG5
, with a surprisingly narrow distribution function contributing with 37(3)% to the R(t), has central V
and
EFG parameters which are quite close to the previously observed EFG5. Both EFG5
and EFG6 bear similar V
parameters: 19.58(4) and 19.31(9)
21 V·m
−2, respectively. While nevertheless, both the axial asymmetry parameter
= 0.63(1) differs from
= 0, as well as the relative attenuation parameter
= 1.3(1)% and
= 24(2)%. EFG6 is obviously a representative of a set of
Cd probes at a particular local defect configuration with axial symmetry
= 0, which is located in places with important density of randomly distributed defects. Even though it is not a trivial task to make analogies, the spectral shape resembles results previously observed in other hydrogenated semiconductors (GaP:H, InP:H and InSb:H) [
28]. Moreover, it has been argued that Cd-H pairs in these semiconductors generally have
= 0, which could hint on the origin of EFG6 observed at this temperature.
3.5. Hydrogenated at 573 K
Following hydrogenation at 573 K,
Cd implantation and subsequent measurement at 581 K, there is obvious evidence for the remnants of EFG5
, with a slightly decreased fraction, now designated as EFG5
. Undoubtedly, the defect interacting with the Cd at the origin of EFG6, characterised by V
= 19.31(9)
21 V·m
−2,
= 0 and
= 24(4)% is gone. A new predominant EFG6
, which still has a wide distribution and characterised with V
19.85(4)
21 V·m
−2,
= 0.57(2) and
= 15(2)% appears. Noteworthy is the fact that both Cd-defect configurations, EFG5
and EFG6
, evolve now in places with lower concentration of randomly distributed defects (see
Table 1). From IR studies performed by Lavrov et al., one could think that hydrogen could remain in the lattice up to 1173 K, if stabilised by an impurity or native defect in the local surrounding [
6]. This might be only one factor explaining the lack of time dynamic effects observable in the R(t) spectra, due to H dynamics and trapping/de-trapping of H at the Cd probe atoms.
The presence of H-related defects and the further interactions with the Cd probe atoms do look highly stable at the measuring temperatures. These are probably complex defects, that act as deep traps for hydrogen and the Cd related probe atom, leading to no observable dynamics on the atomic scale under the present experimental conditions. Nonetheless, one can be convinced that partial implantation damage annealing is happening within the first half an hour of the measurement, the actual relevance of the diluted concentration of Cd and associated defects on the present set of experiments, looks very much irrelevant, regarding the hydrogenation (damage and doping) effects for all processing temperatures.
3.6. Hydrogenated at 663 K
Hydrogenation at 663 K, with a following implantation of
Cd probes and subsequent measurement at 573 K presents quite unexpected results. There are three different EFG distributions, with almost no traces of the previous Cd configurations, therefore we had to evaluate the data in the best feasible way due to a relatively damped spectrum and several possible ways to approach the data. Among the EFGs, there is the only one that resembles an already measured EFG, is EFG5
, although accounting for a reduced fraction of 10(3)%. However, EFG5
could be of different nature, since diffusion of hydrogen is favourable at such hydrogenation temperatures as well as heavy reduction of the upper layers. This assertion could lead to formation of various non-stoichiometric phases (e.g., Magnéli phases or interstitial Ti). As the PAC measurements performed in vacuum, a chance of obtaining homogenous stoichiometric structure back is unlikely. Because of this, one may think that the temperature of hydrogenation was too high, seriously degrading the sample’s upper layers with no possible recovery (e.g., turning them slightly amorphous). Regardless, results obtained by GIXRD measurements show no evidence of such effect (see
Figure 2), and moreover demonstrate a shifting trend of the (110) reflection throughout treatments in the left-hand side.
The latter, was observed in ZnO doped with hydrogen and was attributed to hydrogen being incorporated into the lattice [
29]. Based on the observed frequencies, one may assume that the family of observed EFG5 components are due to a combined complex, where one Cd atom is surrounded by the apical vacancy [
24] and hydrogen. In order to perceive the exact location and chemical nature of the unique configuration, one needs to perform various DFT calculations. Preliminary calculations with the same supercell parameters as in Ref. [
24], but including a substitution of V
o with H were proved to be inadequate, employing the standard PAW method in
vasp environment. Therefore, performing large-scale quantum molecular dynamics simulations and explore other possible defects configurations are two ways to further investigate the systems under the scope [
30].