A Phosphonic Acid Anchoring Analogue of the Sensitizer P1 for p-Type Dye-Sensitized Solar Cells
Abstract
:1. Introduction
2. Materials and Methods
2.1. General
2.2. Compound 1
2.3. Compound 2
2.4. Compound 3
2.5. Compound 4
2.6. PP1
2.7. Crystallography
2.8. Electrode Preparation
2.9. Solar Cell Measurements
2.10. Electrochemical Impedance Spectroscopy (EIS), Open-Circuit Photovoltage Decay (OCVD) and Intensity-Modulated Photocurrent Spectroscopy (IMPS) Measurements
3. Results and Discussion
3.1. Synthesis and Characterization of PP1
3.2. Crystal Structure of Intermediate 3
3.3. Electrode Preparation
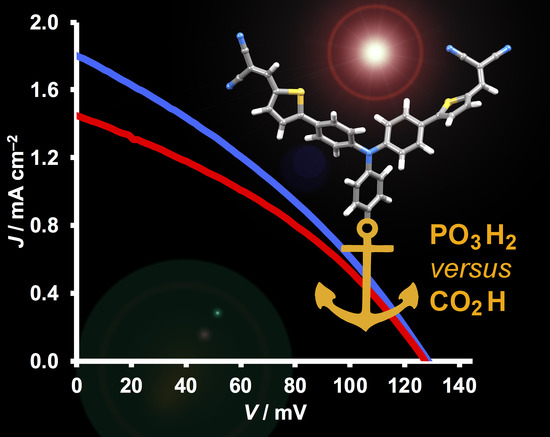
3.4. DSC Performances
3.5. Electrochemical Impedance Spectroscopy (EIS)
3.6. Open-Circuit Photovoltage Decay (OCVD) and Intensity-Modulated Photocurrent Spectroscopy (IMPS) Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- O’Reagan, B.; Grätzel, M. A low-cost, high-efficiency solar cell based on dye-sensitized colloidal TiO2 films. Nature 1991, 353, 737–740. [Google Scholar] [CrossRef]
- Nazeeruddin, Md.K.; Baranoff, E.; Grätzel, M. Dye-sensitized solar cells. A brief overview. Sol. Energy 2011, 85, 1172–1178. [Google Scholar] [CrossRef]
- Grätzel, M. Recent Advances in Sensitized Mesoscopic Solar Cells. Acc. Chem. Res. 2009, 42, 1788–1798. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Solar energy conversion by dye-sensitized photovoltaic cells. Inorg. Chem. 2005, 44, 6841–6851. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Cole, J.M. Anchoring groups for dye-sensitized solar cells. ACS Appl. Mater. Interfaces 2015, 7, 3427–3455. [Google Scholar] [CrossRef] [PubMed]
- Stephens, A.J.; Malzner, F.J.; Constable, E.C.; Housecroft, C.E. The influence of phosphonic acid protonation state on the efficiency of bis(diimine)copper(I) dye-sensitized solar cells. Sustain. Energy Fuels 2018, 2, 786–794. [Google Scholar] [CrossRef]
- Odobel, F.; Pellegrin, Y.; Gibson, E.A.; Hagfeldt, A.; Smeigh, A.L.; Hammarström, L. Recent advances and future directions to optimize the performance of dye-sensitized solar cells. Coord. Chem. Rev. 2012, 256, 2414–2423. [Google Scholar] [CrossRef]
- Nikolaou, V.; Charisiadis, A.; Charalambidis, G.; Coutsolelos, A.G.; Odobel, F. Recent advances and insights in dye-sensititzed NiO photocathodes for photovoltaic devices. J. Mater. Chem. A 2017, 5, 21077–21113. [Google Scholar] [CrossRef]
- Odobel, F.; Le Pleux, L.; Pellegrin, Y.; Blart, E. New photovoltaic devices based on the sensitization of p-type semiconductors: Challenges and opportunities. Acc. Chem. Res. 2010, 43, 1063–1071. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Natu, G.; Ji, Z.; He, M.; Yu, M.; Wu, Y. Probing the Low Fill Factor of NiO p-Type Dye-Sensitized Solar Cells. J. Phys. Chem. C 2012, 116, 26239–26246. [Google Scholar] [CrossRef]
- Borgström, M.; Blart, E.; Boschloo, G.; Mukhtar, E.; Hagfeldt, A.; Hammarström, L.; Odobel, F. Sensitized Hole Injection of Phosphorus Porphyrin into NiO: Toward New Photovoltaic Devices. J. Phys. Chem. B 2005, 109, 22928–22934. [Google Scholar] [CrossRef] [PubMed]
- Yan, W.; Chaitanya, K.; Sun, Z.-D.; Ju, X.-H. Theoretical study on p-type D-π-A snsitizers with modified π-spacers for dye-sensitized solar cells. J. Mol. Model. 2018, 24, 68. [Google Scholar] [CrossRef] [PubMed]
- Piccinin, S.; Rocca, D.; Pastore, M. Role of solvent in the energy level alignment of dye-sensitized NiO interfaces. J. Phys. Chem. C 2017, 121, 22286–22294. [Google Scholar] [CrossRef]
- Muñoz-García, A.B.; Pavone, M. Structure and energy level alignment at the dye-electrode interface in p-type DSSCs: New hints on the role of anchoring modes from ab initio calculations. Phys. Chem. Phys. Chem. 2015, 17, 12238–12246. [Google Scholar] [CrossRef] [PubMed]
- Wykes, M.; Odobel, F.; Adamo, C.; Ciofini, I.; Labat, F. Anchoring groups for dyes in p-DSSC application: Insights from DFT. J. Mol. Model. 2016, 22, 289. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Favereau, L.; Farre, Y.; Maufroy, A.; Pellegrin, Y.; Blart, E.; Hissler, M.; Jacquemin, D.; Odobel, F.; Hammarström, L. Molecular-structure control of electron transfer dynamics of push-pull porphyrins as sensitizers for NiO based dye sensitized solar cells. RSC Adv. 2016, 6, 77184–77194. [Google Scholar] [CrossRef]
- Qin, P.; Zhu, H.; Edvinsson, T.; Boschloo, G.; Hagfeldt, A.; Sun, L. Design of an organic chromophore for p-type dye-sensititzed solar cells. J. Am. Chem. Soc. 2008, 130, 8570–8571. [Google Scholar] [CrossRef] [PubMed]
- Wood, C.J.; Summers, G.H.; Clark, C.A.; Kaeffer, N.; Braeutigam, M.; Carbone, L.R.; D’Amario, L.; Fan, K.; Farre, Y.; Narbey, S.; et al. A comprehensive comparison of dye-sensitized NiO photocathodes for solar energy conversion. Phys. Chem. Chem. Phys. 2016, 18, 10727–10738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Click, K.A.; Beauchamp, D.R.; Garrett, B.R.; Huang, Z.; Hadad, C.M.; Wu, Y. A double-acceptor as a superior organic dye design for p-type DSSCs: High photocurrents and the observed light soaking effect. Phys. Chem. Chem. Phys. 2014, 16, 26103–26111. [Google Scholar] [CrossRef] [PubMed]
- Pellegrin, Y.; Le Pleux, L.; Blart, E.; Renaud, A.; Chavillon, B.; Szuwarski, N.; Boujita, M.; Cario, L.; Jobic, S.; Jacquemin, D.; et al. Ruthenium polypyridine complexes as sensitizers in NiO based p-type dye-sensitized solar cells: Effects of the anchoring groups. J. Photochem. Photobiol. A 2011, 219, 235–242. [Google Scholar] [CrossRef]
- Marinakis, N.; Wobill, C.; Constable, E.C.; Housecroft, C.E. Refining the anchor: Optimizing the performance of cyclometallated ruthenium(II) dyes in p-type dye sensitized solar cells. Polyhedron 2018, 140, 122–128. [Google Scholar] [CrossRef]
- Brunner, F.; Marinakis, N.; Wobill, C.; Willgert, M.; Ertl, C.D.; Kosmalski, T.; Neuburger, M.; Bozic-Weber, B.; Glatzel, T.; Constable, E.C.; et al. Modular synthesis of simple cycloruthenated complexes with state-of-the-art performance in p-type DSCs. J. Mater. Chem. C 2016, 4, 9823–9833. [Google Scholar] [CrossRef] [Green Version]
- Marinakis, N.; Willgert, M.; Constable, E.C.; Housecroft, C.E. Optimization of performance and long-term stability of p-type dye-sensitized solar cells with a cycloruthenated dye through electrolyte solvent tuning. Sustain. Energy Fuels 2017, 1, 626–635. [Google Scholar] [CrossRef] [Green Version]
- Bruker X-ray Diffraction Laboratory. M86-E01078 APEX2 User Manual, 2nd ed.; Bruker AXS Inc.: Madison, WI, USA, 2006. [Google Scholar]
- Palatinus, L.; Chapuis, G. SUPERFLIP. A computer program for the solution of crystal structures by charge flipping in arbitrary dimensions. J. Appl. Cryst. 2007, 40, 786–790. [Google Scholar] [CrossRef]
- Betteridge, P.W.; Carruthers, J.R.; Cooper, R.I.; Prout, K.; Watkin, D.J. CRYSTALS Version 12: Software for Guided Crystal Structure Analysis. J. Appl. Cryst. 2003, 36, 1487–1487. [Google Scholar] [CrossRef]
- Macrae, C.F.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Shields, G.P.; Taylor, R.; Towler, M.; van de Streek, J. Mercury: Visualization and analysis of crystal structures. J. Appl. Cryst. 2006, 39, 453–457. [Google Scholar] [CrossRef]
- Macrae, C.F.; Bruno, I.J.; Chisholm, J.A.; Edgington, P.R.; McCabe, P.; Pidcock, E.; Rodriguez-Monge, L.; Taylor, R.; van de Streek, J.; Wood, P.A. Mercury CSD 2.0—New Features for the Visualization and Investigation of Crystal Structures. J. Appl. Cryst. 2008, 41, 466–470. [Google Scholar] [CrossRef]
- Hod, I.; Tachan, Z.; Shalom, M.; Zaban, A. Characterization and control of the electronic properties of a NiO based dye sensitized photocathode. Phys. Chem. Chem. Phys. 2013, 15, 6339–6343. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Huang, F.; Nattestad, A.; Wang, K.; Fu, D.; Mishra, A.; Bäuerle, P.; Bach, U.; Cheng, Y.-B. Enhanced open-circuit voltage of p-type DSC with highly crystalline NiO nanoparticles. Chem. Commun. 2011, 47, 4808–4810. [Google Scholar] [CrossRef] [PubMed]
- Perera, I.R.; Daeneke, T.; Makuta, S.; Yu, Z.; Tachibana, Y.; Mishra, A.; Bäuerle, P.; Ohlin, C.A.; Bach, U.; Spiccia, L. Application of the tris(acetlyacetonato)iron(III)/(II) redox couple in p-type dye-sensitized solar cells. Angew. Chem. Int. Ed. 2015, 54, 3758–3762. [Google Scholar] [CrossRef] [PubMed]
- Farré, Y.; Raissi, M.; Fihey, A.; Pellegrin, Y.; Blart, E.; Jacquemin, D.; Odobel, F. A blue diketopyrrolopyrrole sensitizer with high efficiency in nickel-oxide-based dye-sensitized solar cells. ChemSusChem 2017, 10, 2618–2625. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gibson, E.A.; Qin, P.; Boschloo, G.; Gorlov, M.; Hagfeldt, A.; Sun, L. Double-layered NiO photocathodes for p-type DSSCs with record IPCE. Adv. Mater. 2010, 22, 1759–1762. [Google Scholar] [CrossRef] [PubMed]
- Bisquert, J. Theory of the impedance of charge transfer via surface states in dye-sensitized solar cells. J. Electroanal. Chem. 2010, 646, 43–51. [Google Scholar] [CrossRef]
- Fabregat-Santiago, F.; Garcia-Belmonte, G.; Mora-Seró, I.; Bisquert, J. Characterization of nanostructured hybrid and organic solar cells by impedance spectroscopy. Phys. Chem. Chem. Phys. 2011, 13, 9083–9118. [Google Scholar] [CrossRef] [PubMed]
- Fabregat-Santiago, F.; Bisquert, J.; Palomares, E.; Otero, L.; Kuang, D.; Zakeeruddin, S.M.; Grätzel, M. Correlation between photovoltaic performance and impedance spectroscopy of dye-sensiitzed solar cells based on ionic liquids. J. Phys. Chem. C 2007, 111, 6550–6560. [Google Scholar] [CrossRef]
- Bisquert, J.; Garcia-Belmonte, G.; Fabregat-Santiago, F.; Compte, A. Anomalous transport effects in the impedance of porous film electrodes. Electrochem. Commun. 1999, 1, 429–435. [Google Scholar] [CrossRef]
- Córdoba-Torres, P. Relationship between constant-phase element (CPE) parameters and physical properties of films with distributed resistivity. Electrochim. Acta 2017, 225, 592–604. [Google Scholar] [CrossRef]
- Ho, P.; Bao, L.Q.; Ahn, K.S.; Cheruku, R.; Kim, J.H. P-Type dye-sensitized solar cells: Enhanced performance with a NiO compact blocking layer. Synth. Met. 2016, 217, 314–321. [Google Scholar] [CrossRef]
- Shoar Abouzari, M.R.; Berkemeier, F.; Schmitz, G.; Wilmer, D. On the physical interpretation of constant phase elements. Solid State Ionics 2009, 180, 922–927. [Google Scholar] [CrossRef]
- Bisquert, J.; Zaban, A.; Greenshtein, M.; Mora-Seró, I. Determination of rate constants for charge transfer and the distribution of semiconductor and electrolyte electronic energy levels in dye-sensitized solar cells by open-circuit photovoltage decay method. J. Am. Chem. Soc. 2004, 126, 13550–13559. [Google Scholar] [CrossRef] [PubMed]
- Zaban, A.; Greenshtein, M.; Bisquert, J. Determination of the electron lifetime in nanocrystalline dye solar cells by open-circuit voltage decay measurements. ChemPhysChem 2003, 4, 859–864. [Google Scholar] [CrossRef] [PubMed]
- Dunn, H.K.; Westin, P.-O.; Staff, D.R.; Peter, L.M.; Walker, A.B.; Boschloo, G.; Hagfeldt, A. Determination of the electron diffusion length in dye-sensitized solar cells by substrate contact patterning. J. Phys. Chem. C 2011, 115, 13932–13937. [Google Scholar] [CrossRef] [Green Version]
- Schlichthörl, G.; Huang, S.Y.; Sprague, J.; Frank, A.J. Band edge movement and recombination kinetics in dye-sensitized nanocrystalline TiO2 solar cells: A study by intensity modulated photovoltage spectroscopy. J. Phys. Chem. B 1997, 101, 8141–8155. [Google Scholar] [CrossRef]
- Dloczik, L.; Ileperuma, O.; Lauermann, I.; Peter, L.M.; Ponomarev, E.A.; Redmond, G.; Shaw, N.J.; Uhlendorf, I. Dynamic response of dye-sensitized nanocrystalline solar cells: Characterization by intensity-modulated photocurrent spectroscopy. J. Phys. Chem. B 1997, 101, 10281–10289. [Google Scholar] [CrossRef]
- Zhu, H.; Hagfeldt, A.; Boschloo, G. Photoelectrochemistry of mesoporous NiO electrodes in iodide/triiodide electrolytes. J. Phys. Chem. C 2007, 111, 17455–17458. [Google Scholar] [CrossRef]
- Peter, L. Transport, trapping and interfacial transfer of electrons in dye-sensitized nanocrystalline solar cells. J. Electroanal. Chem. 2007, 599, 233–240. [Google Scholar] [CrossRef]
- Abate, A.; Pérez-Tejadam, R.; Wojciechowski, K.; Foster, J.M.; Sadhanala, A.; Steiner, U.; Snaith, H.J.; Franco, S.; Orduna, J. Phosphonic anchoring groups in organic dyes for solid-state solar cells. Phys. Chem. Chem. Phys. 2015, 17, 18780–18789. [Google Scholar] [CrossRef] [PubMed] [Green Version]















| Dye | DSC Number | JSC/mA cm−2 | VOC/mV | ff/% | η/% |
|---|---|---|---|---|---|
| P1 | 1 | 1.55 | 130 | 34 | 0.068 |
| P1 | 2 | 1.80 | 128 | 33 | 0.076 |
| P1 | 3 | 1.74 | 130 | 35 | 0.079 |
| P1 | 4 | 1.64 | 125 | 34 | 0.071 |
| P1 | 5 | 1.76 | 117 | 32 | 0.065 |
| PP1 | 1 | 1.45 | 127 | 35 | 0.065 |
| PP1 | 2 | 1.45 | 132 | 36 | 0.069 |
| PP1 | 3 | 1.32 | 119 | 34 | 0.054 |
| PP1 | 4 | 1.43 | 122 | 34 | 0.059 |
| PP1 | 5 | 1.11 | 143 | 34 | 0.054 |
| Rs/Ω | RPt/Ω | CPt/µF | Rtr/Ω | Rrec/Ω | Cμ/μF | αa | |
|---|---|---|---|---|---|---|---|
| P1 | 17.7 | 4.8 | 7.9 | 1.9 | 507.1 | 485.6 | 0.70 |
| PP1 | 11.1 | 2.0 | 20 | 8.3 | 729.9 | 96.5 | 0.88 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klein, Y.M.; Marinakis, N.; Constable, E.C.; Housecroft, C.E. A Phosphonic Acid Anchoring Analogue of the Sensitizer P1 for p-Type Dye-Sensitized Solar Cells. Crystals 2018, 8, 389. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst8100389
Klein YM, Marinakis N, Constable EC, Housecroft CE. A Phosphonic Acid Anchoring Analogue of the Sensitizer P1 for p-Type Dye-Sensitized Solar Cells. Crystals. 2018; 8(10):389. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst8100389
Chicago/Turabian StyleKlein, Y. Maximilian, Nathalie Marinakis, Edwin C. Constable, and Catherine E. Housecroft. 2018. "A Phosphonic Acid Anchoring Analogue of the Sensitizer P1 for p-Type Dye-Sensitized Solar Cells" Crystals 8, no. 10: 389. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst8100389