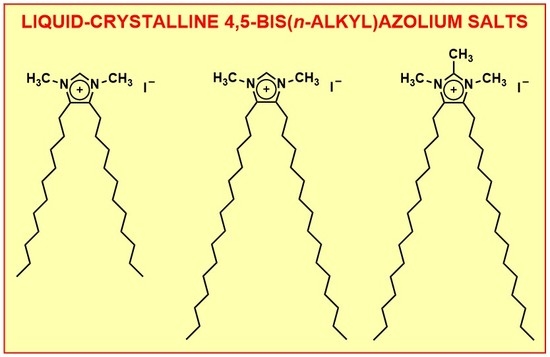
Substituted Azolium Disposition: Examining the Effects of Alkyl Placement on Thermal Properties
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bowlas, C.J.; Bruce, D.W.; Seddon, K.R. Liquid-Crystalline Ionic Liquids. Chem. Commun. 1996, 1625–1626. [Google Scholar] [CrossRef]
- Gordon, C.M.; Holbrey, J.D.; Kennedy, A.R.; Seddon, K.R. Ionic Liquid Crystals: Hexafluorophosphate Salts. J. Mater. Chem. 1998, 8, 2627–2636. [Google Scholar] [CrossRef]
- Holbrey, J.D.; Seddon, K.R. The Phase Behaviour of 1-Alkyl-3-Methylimidazolium Tetrafluoroborates; Ionic Liquids and Ionic Liquid Crystals. J. Chem. Soc. Dalton Trans. 1999, 2133–2139. [Google Scholar] [CrossRef]
- Goossens, K.; Lava, K.; Nockemann, P.; Van Hecke, K.; Van Meervelt, L.; Driesen, K.; Görller-Walrand, C.; Binnemans, K.; Cardinaels, T. Pyrrolidinium Ionic Liquid Crystals. Chem. Eur. J. 2009, 15, 656–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Mansueto, M.; Laschat, S. Ionic Liquid Crystals. In Handbook of Liquid Crystals. Volume 6: Nanostructured and Amphiphilic Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 231–280. [Google Scholar]
- Fernandez, A.A.; Kouwer, P.H.J. Key Developments in Ionic Liquid Crystals. Int. J. Mol. Sci. 2016, 17, 731. [Google Scholar] [CrossRef] [PubMed]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of Ions and Electrons in Nanostructured Liquid Crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Kato, T.; Uchida, J.; Ichikawa, T.; Sakamoto, T. Functional Liquid Crystals towards the Next Generation of Materials. Angew. Chem. Int. Ed. 2018, 57, 4355–4371. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Ionic Liquid Crystal as a Hole Transport Layer of Dye-Sensitized Solar Cells. Chem. Commun. 2005, 740–742. [Google Scholar] [CrossRef]
- Yamanaka, N.; Kawano, R.; Kubo, W.; Masaki, N.; Kitamura, T.; Wada, Y.; Watanabe, M.; Yanagida, S. Dye-Sensitized TiO2 Solar Cells Using Imidazolium-Type Ionic Liquid Crystal Systems As Effective Electrolytes. J. Phys. Chem. B 2007, 111, 4763–4769. [Google Scholar] [CrossRef]
- Högberg, D.; Soberats, B.; Yatagai, R.; Uchida, S.; Yoshio, M.; Kloo, L.; Segawa, H.; Kato, T. Liquid-Crystalline Dye-Sensitized Solar Cells: Design of Two-Dimensional Molecular Assemblies for Efficient Ion Transport and Thermal Stability. Chem. Mater. 2016, 28, 6493–6500. [Google Scholar] [CrossRef]
- Bruce, D.W.; Gao, Y.A.; Canongia Lopes, J.N.; Shimizu, K.; Slattery, J.M. Liquid-Crystalline Ionic Liquids As Ordered Reaction Media for the Diels-Alder Reaction. Chem. Eur. J. 2016, 22, 16113–16123. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Funahashi, M.; Kato, T. An Electrochromic Nanostructured Liquid Crystal Consisting of π-Conjugated and Ionic Moieties. J. Am. Chem. Soc. 2008, 130, 13206–13207. [Google Scholar] [CrossRef] [PubMed]
- Yazaki, S.; Funahashi, M.; Kagimoto, J.; Ohno, H.; Kato, T. Nanostructured Liquid Crystals Combining Ionic and Electronic Functions. J. Am. Chem. Soc. 2010, 132, 7702–7708. [Google Scholar] [CrossRef] [PubMed]
- Beneduci, A.; Cospito, S.; La Deda, M.; Veltri, L.; Chidichimo, G. Electrofluorochromism in π-Conjugated Ionic Liquid Crystals. Nat. Commun. 2014, 5, 3105. [Google Scholar] [CrossRef] [PubMed]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed]
- Ji, Y.; Shi, R.; Wang, Y.; Saielli, G. Effect of the Chain Length on the Structure of Ionic Liquids: From Spatial Heterogeneity to Ionic Liquid Crystals. J. Phys. Chem. B 2013, 117, 1104–1109. [Google Scholar] [CrossRef]
- Nemoto, F.; Kofu, M.; Yamamuro, O. Thermal and Structural Studies of Imidazolium-Based Ionic Liquids with and Without Liquid-Crystalline Phases: The Origin of Nanostructure. J. Phys. Chem. B 2015, 119, 5028–5034. [Google Scholar] [CrossRef]
- Russina, O.; Lo Celso, F.; Plechkova, N.; Jafta, C.J.; Appetecchi, G.B.; Triolo, A. Mesoscopic Organization in Ionic Liquids. Top. Curr. Chem. 2017, 375, 58. [Google Scholar] [CrossRef]
- Russina, O.; Lo Celso, F.; Plechkova, N.V.; Triolo, A. Emerging Evidences of Mesoscopic-Scale Complexity in Neat Ionic Liquids and Their Mixtures. J. Phys. Chem. Lett. 2017, 8, 1197–1204. [Google Scholar] [CrossRef]
- Bruce, D.W.; Cabry, C.P.; Canongia Lopes, J.N.; Costen, M.L.; D’Andrea, L.; Grillo, I.; Marshall, B.C.; McKendrick, K.G.; Minton, T.K.; Purcell, S.M.; et al. Nanosegregation and Structuring in the Bulk and at the Surface of Ionic-Liquid Mixtures. J. Phys. Chem. B 2017, 121, 6002–6020. [Google Scholar] [CrossRef] [PubMed]
- Pontoni, D.; Haddad, J.; Di Michiel, M.; Deutsch, M. Self-Segregated Nanostructure in Room Temperature Ionic Liquids. Soft Matter 2017, 13, 6947–6955. [Google Scholar] [CrossRef]
- Cosby, T.; Vicars, Z.; Wang, Y.; Sangoro, J. Dynamic-Mechanical and Dielectric Evidence of Long-Lived Mesoscale Organization in Ionic Liquids. J. Phys. Chem. Lett. 2017, 8, 3544–3548. [Google Scholar] [CrossRef] [PubMed]
- Nemoto, F.; Kofu, M.; Nagao, M.; Ohishi, K.; Takata, S.; Suzuki, J.; Yamada, T.; Shibata, K.; Ueki, T.; Kitazawa, Y.; et al. Neutron Scattering Studies on Short- and Long-Range Layer Structures and Related Dynamics in Imidazolium-Based Ionic Liquids. J. Chem. Phys. 2018, 149, 054502. [Google Scholar] [CrossRef] [PubMed]
- Cabry, C.P.; D’Andrea, L.; Shimizu, K.; Grillo, I.; Li, P.; Rogers, S.; Bruce, D.W.; Canongia Lopes, J.N.; Slattery, J.M. Exploring the Bulk-Phase Structure of Ionic Liquid Mixtures Using Small-Angle Neutron Scattering. Faraday Discuss. 2018, 206, 265–289. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.D.; Wang, Y.T.; Saielli, G. Metastable State During Melting and Solid-Solid Phase Transition of [CnMim][NO3] (n = 4-12) Ionic Liquids by Molecular Dynamics Simulation. J. Phys. Chem. B 2018, 122, 229–239. [Google Scholar] [CrossRef] [PubMed]
- Douce, L.; Suisse, J.-M.; Guillon, D.; Taubert, A. Imidazolium-Based Liquid Crystals: A Modular Platform for Versatile New Materials with Finely Tuneable Properties and Behaviour. Liq. Cryst. 2011, 38, 1653–1661. [Google Scholar] [CrossRef]
- Bara, J.E.; Shannon, M.S. Beyond 1,3-Difunctionalized Imidazolium Cations. Nanomater. Energy 2012, 1, 237–242. [Google Scholar] [CrossRef]
- Scalfani, V.F.; Alshaikh, A.A.; Bara, J.E. Analysis of the Frequency and Diversity of 1,3-Dialkylimidazolium Ionic Liquids Appearing in the Literature. Ind. Eng. Chem. Res. 2018, 57, 15971–15981. [Google Scholar] [CrossRef]
- Fox, D.M.; Awad, W.H.; Gilman, J.W.; Maupin, P.H.; De Long, H.C.; Trulove, P.C. Flammability, Thermal Stability, and Phase Change Characteristics of Several Trialkylimidazolium Salts. Green Chem. 2003, 5, 724–727. [Google Scholar] [CrossRef]
- Mukai, T.; Yoshio, M.; Kato, T.; Ohno, H. Effect of Methyl Groups Onto Imidazolium Cation Ring on Liquid Crystallinity and Ionic Conductivity of Amphiphilic Ionic Liquids. Chem. Lett. 2004, 33, 1630–1631. [Google Scholar] [CrossRef]
- Mukai, T.; Yoshio, M.; Kato, T.; Yoshizawa-Fujita, M.; Ohno, H. Self-Organization of Protonated 2-Heptadecylimidazole as an Effective Ion Conductive Matrix. Electrochemistry 2005, 73, 623–626. [Google Scholar]
- Kouwer, P.H.J.; Swager, T.M. Synthesis and Mesomorphic Properties of Rigid-Core Ionic Liquid Crystals. J. Am. Chem. Soc. 2007, 129, 14042–14052. [Google Scholar] [CrossRef] [PubMed]
- Yoshio, M.; Ichikawa, T.; Shimura, H.; Kagata, T.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Columnar Liquid-Crystalline Imidazolium Salts. Effects of Anions and Cations on Mesomorphic Properties and Ionic Conductivity. Bull. Chem. Soc. Jpn. 2007, 80, 1836–1841. [Google Scholar] [CrossRef]
- Alam, M.A.; Motoyanagi, J.; Yamamoto, Y.; Fukushima, T.; Kim, J.; Kato, K.; Takata, M.; Saeki, A.; Seki, S.; Tagawa, S.; et al. “Bicontinuous Cubic” Liquid Crystalline Materials from Discotic Molecules: A Special Effect of Paraffinic Side Chains with Ionic Liquid Pendants. J. Am. Chem. Soc. 2009, 131, 17722–17723. [Google Scholar] [CrossRef] [PubMed]
- Li, C.H.; He, J.H.; Liu, J.H.; Qian, L.A.; Yu, Z.Q.; Zhang, Q.L.; He, C.X. Liquid Crystalline Phases of 1,2-Dimethyl-3-Hexadecylimidazolium Bromide and Binary Mixtures With Water. J. Colloid Interface Sci. 2010, 349, 224–229. [Google Scholar] [CrossRef]
- Goossens, K.; Wellens, S.; Van Hecke, K.; Van Meervelt, L.; Cardinaels, T.; Binnemans, K. T-Shaped Ionic Liquid Crystals Based on the Imidazolium Motif: Exploring Substitution of the C-2 Imidazolium Carbon Atom. Chem. Eur. J. 2011, 17, 4291–4306. [Google Scholar] [CrossRef]
- Fernandez, A.A.; de Haan, L.T.; Kouwer, P.H.J. Towards Room-Temperature Ionic Liquid Crystals. J. Mater. Chem. A 2013, 1, 354–357. [Google Scholar] [CrossRef]
- Nestor, S.T.; Heinrich, B.; Sykora, R.A.; Zhang, X.; McManus, G.J.; Douce, L.; Mirjafari, A. Methimazolium-Based Ionic Liquid Crystals: Emergence of Mesomorphic Properties via a Sulfur Motif. Tetrahedron 2017, 73, 5456–5460. [Google Scholar] [CrossRef]
- Hindman, M.S.; Stanton, A.D.; Christopher Irvin, A.; Wallace, D.A.; Moon, J.D.; Reclusado, K.R.; Liu, H.; Belmore, K.A.; Liang, Q.; Shannon, M.S.; et al. Synthesis of 1,2-Dialkyl-, 1,4(5)-Dialkyl-, and 1,2,4(5)-Trialkylimidazoles via a One-Pot Method. Ind. Eng. Chem. Res. 2013, 52, 11880–11887. [Google Scholar] [CrossRef]
- Yue, S.; Roveda, J.D.; Mittenthal, M.S.; Shannon, M.S.; Bara, J.E. Experimental Densities and Calculated Fractional Free Volumes of Ionic Liquids With Tri- and Tetra-Substituted Imidazolium Cations. J. Chem. Eng. Data 2018, 63, 2522–2532. [Google Scholar] [CrossRef]
- Fumino, K.; Wulf, A.; Ludwig, R. Strong, Localized, and Directional Hydrogen Bonds Fluidize Ionic Liquids. Angew. Chem. Int. Ed. 2008, 47, 8731–8734. [Google Scholar] [CrossRef] [PubMed]
- Fumino, K.; Wulf, A.; Ludwig, R. The Potential Role of Hydrogen Bonding in Aprotic and Protic Ionic Liquids. Phys. Chem. Chem. Phys. 2009, 11, 8790–8794. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Peppel, T.; Fumino, K.; Köckerling, M.; Ludwig, R. The Importance of Hydrogen Bonds for the Structure of Ionic Liquids: Single-Crystal X-ray Diffraction and Transmission and Attenuated Total Reflection Spectroscopy in the Terahertz Region. Angew. Chem. Int. Ed. 2010, 49, 10221–10224. [Google Scholar] [CrossRef] [PubMed]
- Wulf, A.; Fumino, K.; Ludwig, R. Spectroscopic Evidence for an Enhanced Anion-Cation Interaction from Hydrogen Bonding in Pure Imidazolium Ionic Liquids. Angew. Chem. Int. Ed. 2010, 49, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Peppel, T.; Roth, C.; Fumino, K.; Paschek, D.; Köckerling, M.; Ludwig, R. The Influence of Hydrogen-Bond Defects on the Properties of Ionic Liquids. Angew. Chem. Int. Ed. 2011, 50, 6661–6665. [Google Scholar] [CrossRef] [PubMed]
- Fumino, K.; Peppel, T.; Geppert-Rybczynska, M.; Zaitsau, D.H.; Lehmann, J.K.; Verevkin, S.P.; Köckerling, M.; Ludwig, R. The Influence of Hydrogen Bonding on the Physical Properties of Ionic Liquids. Phys. Chem. Chem. Phys. 2011, 13, 14064–14075. [Google Scholar] [CrossRef]
- Hugar, K.M.; Kostalik, H.A.; Coates, G.W. Imidazolium Cations with Exceptional Alkaline Stability: A Systematic Study of Structure-Stability Relationships. J. Am. Chem. Soc. 2015, 137, 8730–8737. [Google Scholar] [CrossRef]
- Wang, D.; Richter, C.; Rühling, A.; Drücker, P.; Siegmund, D.; Metzler-Nolte, N.; Glorius, F.; Galla, H.-J. A Remarkably Simple Class of Imidazolium-Based Lipids and Their Biological Properties. Chem. Eur. J. 2015, 21, 15123–15126. [Google Scholar] [CrossRef]
- Rühling, A.; Wang, D.; Ernst, J.B.; Wulff, S.; Honeker, R.; Richter, C.; Ferry, A.; Galla, H.-J.; Glorius, F. Influence of the Headgroup of Azolium-Based Lipids on Their Biophysical Properties and Cytotoxicity. Chem. Eur. J. 2017, 23, 5920–5924. [Google Scholar] [CrossRef]
- Drücker, P.; Rühling, A.; Grill, D.; Wang, D.; Draeger, A.; Gerke, V.; Glorius, F.; Galla, H.-J. Imidazolium Salts Mimicking the Structure of Natural Lipids Exploit Remarkable Properties Forming Lamellar Phases and Giant Vesicles. Langmuir 2017, 33, 1333–1342. [Google Scholar] [CrossRef] [PubMed]
- Rakers, L.; Glorius, F. Flexible Design of Ionic Liquids for Membrane Interactions. Biophys. Rev. 2018, 10, 747–750. [Google Scholar] [CrossRef] [PubMed]
- Rondla, R.; Lee, C.K.; Lu, J.T.; Lin, I.J.B. Symmetrical 1,3-Dialkylimidazolium Based Ionic Liquid Crystals. J. Chin. Chem. Soc. 2013, 60, 745–754. [Google Scholar]
- Wang, X.; Heinemann, F.W.; Yang, M.; Melcher, B.U.; Fekete, M.; Mudring, A.-V.; Wasserscheid, P.; Meyer, K. A New Class of Double Alkyl-Substituted, Liquid Crystalline Imidazolium Ionic Liquids—A Unique Combination of Structural Features, Viscosity Effects, and Thermal Properties. Chem. Commun. 2009, 7405–7407. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Vogel, C.S.; Heinemann, F.W.; Wasserscheid, P.; Meyer, K. Solid-State Structures of Double-Long-Chain Imidazolium Ionic Liquids: Influence of Anion Shape on Cation Geometry and Crystal Packing. Cryst. Growth Des. 2011, 11, 1974–1988. [Google Scholar] [CrossRef]
- Wang, X.; Sternberg, M.; Kohler, F.T.U.; Melcher, B.U.; Wasserscheid, P.; Meyer, K. Long-Alkyl-Chain-Derivatized Imidazolium Salts and Ionic Liquid Crystals with Tailor-Made Properties. RSC Adv. 2014, 4, 12476–12481. [Google Scholar] [CrossRef]
- Yang, M.; Mallick, B.; Mudring, A.-V. A Systematic Study on the Mesomorphic Behavior of Asymmetrical 1-Alkyl-3-Dodecylimidazolium Bromides. Cryst. Growth Des. 2014, 14, 1561–1571. [Google Scholar] [CrossRef]
- Lee, C.K.; Peng, H.H.; Lin, I.J.B. Liquid Crystals of N,N′-Dialkylimidazolium Salts Comprising Palladium(II) and Copper(II) Ions. Chem. Mater. 2004, 16, 530–536. [Google Scholar] [CrossRef]
- Ngo, H.L.; LeCompte, K.; Hargens, L.; McEwen, A.B. Thermal Properties of Imidazolium Ionic Liquids. Thermochim. Acta 2000, 357, 97–102. [Google Scholar] [CrossRef]
- Park, G.; Goossens, K.; Shin, T.J.; Bielawski, C.W. Dicyanamide Salts That Adopt Smectic, Columnar, or Bicontinuous Cubic Liquid-Crystalline Mesophases. Chem. Eur. J. 2018, 24, 6399–6411. [Google Scholar] [CrossRef]
- Marcos, M.; Giménez, R.; Serrano, J.L.; Donnio, B.; Heinrich, B.; Guillon, D. Dendromesogens: Liquid Crystal Organizations of Poly(Amidoamine) Dendrimers Versus Starburst Structures. Chem. Eur. J. 2001, 7, 1006–1013. [Google Scholar] [CrossRef]
- Fouchet, J.; Douce, L.; Heinrich, B.; Welter, R.; Louati, A. A Convenient Method for Preparing Rigid-Core Ionic Liquid Crystals. Beilstein J. Org. Chem. 2009, 5, No. 51. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, W.; Douce, L.; Heinrich, B. 1-(4-Alkyloxybenzyl)-3-methyl-1H-imidazol-3-ium Organic Backbone: A Versatile Smectogenic Moiety. Beilstein J. Org. Chem. 2009, 5. No. 62. [Google Scholar] [CrossRef] [PubMed]
- Jenkins, H.D.B.; Roobottom, H.K.; Passmore, J.; Glasser, L. Relationships among Ionic Lattice Energies, Molecular (Formula Unit) Volumes, and Thermochemical Radii. Inorg. Chem. 1999, 38, 3609–3620. [Google Scholar] [CrossRef] [PubMed]
- Dzyuba, S.V.; Bartsch, R.A. New Room-Temperature Ionic Liquids with C2-Symmetrical Imidazolium Cations. Chem. Commun. 2001, 16, 1466–1467. [Google Scholar] [CrossRef]
- Goossens, K.; Nockemann, P.; Driesen, K.; Goderis, B.; Görller-Walrand, C.; Van Hecke, K.; Van Meervelt, L.; Pouzet, E.; Binnemans, K.; Cardinaels, T. Imidazolium Ionic Liquid Crystals With Pendant Mesogenic Groups. Chem. Mater. 2008, 20, 157–168. [Google Scholar] [CrossRef]
- Tschierske, C. Liquid Crystal Engineering—New Complex Mesophase Structures and Their Relations to Polymer Morphologies, Nanoscale Patterning and Crystal Engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Taguchi, S.; Liu, F.; Zeng, X.; Ungar, G.; Ohno, H.; Kato, T. Induction of Thermotropic Bicontinuous Cubic Phases in Liquid-Crystalline Ammonium and Phosphonium Salts. J. Am. Chem. Soc. 2012, 134, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Ungar, G.; Liu, F.; Zeng, X. Cubic and Other 3D Thermotropic Liquid Crystal Phases and Quasicrystals. In Handbook of Liquid Crystals. Volume 5: Non-Conventional Liquid Crystals, 2nd ed.; Goodby, J.W., Collings, P.J., Kato, T., Tschierske, C., Gleeson, H., Raynes, P., Eds.; Wiley-VCH: Weinheim, Germany, 2014; pp. 363–436. [Google Scholar]




| Compound | Transition 1 | T (°C) 2 | ΔH (kJ·mol−1) 3 | T1% (°C) 4 | |
|---|---|---|---|---|---|
| 1-7 | Cr → Iso 5 | 26 | 0.7 | n.d. | |
| 1-11 | HR1: | Cr → SmA 6 | 50, 55 6 | 21.6 6 | n.d. |
| SmA → Iso | 77 | 2.3 | |||
| HR2: | g → SmA | ~−14 | – | ||
| SmA → Iso | 76 | 2.2 | |||
| 1-15 | Cr → SmA | 76 | 38.3 | ~181 | |
| SmA → Iso | 88 7 | 0.7 | |||
| 2 | Cr → SmA 8 | 70 8 | – 8 | ~194 | |
| SmA → Iso | 88 7 | 0.5 | |||
| 3 | Cr → Iso 9 | 73 7 | 16.4 | ~135 | |
| 4 | Cr → Iso 9 | 68 7 | 38.1 | ~206 | |
| Cpd. | Type of LC Mesophase | T (°C) | dobs. (Å) 1 | I2 | hkl3 | dcalcd. (Å) 1 | Structural Parameters of the LC Mesophases 4 |
|---|---|---|---|---|---|---|---|
| 1-15 | SmA | 75 | 31.63 | VS (sh) | 001 | 31.68 | d = 31.68 Å |
| (upon | 15.86 | W (sh) | 002 | 15.84 | Vmol ≈ 1072 Å3 | ||
| cooling) | 4.5–4.6 | br | h1 | AM ≈ 67.7 Å2 | |||
| σch ≈ 22.1 Å2 | |||||||
| 2 | SmA | 78 | 32.91 | VS (sh) | 001 | 32.88 | d = 32.88 Å |
| 16.42 | W (sh) | 002 | 16.44 | Vmol ≈ 1098 Å3 | |||
| 4.4–4.5 | br | h1 | AM ≈ 66.8 Å2 | ||||
| σch ≈ 22.2 Å2 |
| Compound 1 | Phase Transition Temperatures (°C) 2 |
|---|---|
| 1-7 | Cr · 26 · Iso |
| 1-11 | HR1: Cr · ~55 3 · SmA · 77 · Iso |
| HR2: g · ~−14 · SmA · 76 · Iso | |
| 1-15 | Cr · 76 · SmA · 88 · Iso |
| 2 | Cr · 70 · SmA · 88 · Iso |
| [C10C10im][I] (5-10) [54] | Cr · < 0 · SmA · 55 · Iso |
| [C12C12im][I] (5-12) [56] | Cr · 40 · SmA · 89 · Iso |
| [C16C16im][I] (5-16) [54] | Cr · 67 · SmA · 147 · Iso |
| [C10C10im][BF4] [57] | Cr · 18 · SmA · 25 · Iso |
| [C12C12im][BF4] [57] | Cr · 50 · SmA · 69 · Iso |
| [C14C14im][BF4] [57] | Cr · 63 · SmA · 106 · Iso |
| [C16C16im][BF4] [57] | Cr · 70 · SmA · 125 · Iso |
| [C10C10im][PF6] [54,66] | Cr · 16 · Iso |
| [C12C12im][PF6] [54,57] | Cr · 45 · Iso |
| [C14C14im][PF6] [54] | Cr · 59 · SmA · 81 · Iso |
| [C16C16im][PF6] [54] | Cr · 68 · SmA · 105 · Iso |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goossens, K.; Rakers, L.; Shin, T.J.; Honeker, R.; Bielawski, C.W.; Glorius, F. Substituted Azolium Disposition: Examining the Effects of Alkyl Placement on Thermal Properties. Crystals 2019, 9, 34. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010034
Goossens K, Rakers L, Shin TJ, Honeker R, Bielawski CW, Glorius F. Substituted Azolium Disposition: Examining the Effects of Alkyl Placement on Thermal Properties. Crystals. 2019; 9(1):34. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010034
Chicago/Turabian StyleGoossens, Karel, Lena Rakers, Tae Joo Shin, Roman Honeker, Christopher W. Bielawski, and Frank Glorius. 2019. "Substituted Azolium Disposition: Examining the Effects of Alkyl Placement on Thermal Properties" Crystals 9, no. 1: 34. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010034