Modification of Structure and Magnetic Properties in Coordination Assemblies Based on [Cu(cyclam)]2+ and [W(CN)8]3−
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of the [Cu(cyclam)](NO3)2·6H2O Precursor Complex
2.2. Synthesis of {[Cu(cyclam)]3[W(CN)8]2·5H2O}n (1·5H2O)
2.3. Synthesis of {[Cu(cyclam)]3[W(CN)8]2}n (1)
2.4. Synthesis of {[CuII(cyclam)(H2O)]2[CuII(cyclam)][WV(CN)8]2}.3H2O (2·3H2O)
2.5. Structure Determination
3. Results and Discussion
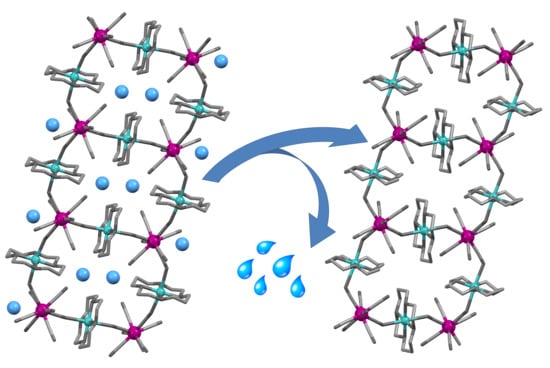
3.1. Synthesis and Post-Synthetic Modification
3.2. Description of Structures
3.3. Magnetic Properties
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Kahn, O.; Larionova, J.; Yakhmi, J.V. Molecular Magnetic Sponges. Chem. Eur. J. 1999, 5, 3443–3449. [Google Scholar] [CrossRef]
- Maspoch, D.; Ruiz-Molina, D.; Wurst, K.; Domingo, N.; Cavallini, M.; Biscarini, F.; Tejada, J.; Rovira, C.; Veciana, J. A nanoporous molecular magnet with reversible solvent-induced mechanical and magnetic properties. Nat. Mater. 2003, 2, 190–195. [Google Scholar] [CrossRef]
- Pinkowicz, D.; Podgajny, R.; Gawel, B.; Nitek, W.; Lasocha, W.; Oszajca, M.; Czapla, M.; Makarewicz, M.; Balanda, M.; Sieklucka, B. Double Switching of a Magnetic Coordination Framework through Intraskeletal Molecular Rearrangement. Angew. Chem. Int. Edit. 2011, 50, 3973–3977. [Google Scholar] [CrossRef]
- Nowicka, B.; Balanda, M.; Gawel, B.; Cwiak, G.; Budziak, A.; Lasocha, W.; Sieklucka, B. Microporous {[Ni(cyclam)]3[W(CN)8]2}n affording reversible structural and magnetic conversions. Dalton Trans. 2011, 40, 3067–3073. [Google Scholar] [CrossRef]
- Nowicka, B.; Rams, M.; Stadnicka, K.; Sieklucka, B. Reversible Guest-Induced Magnetic and Structural Single-Crystal-to-Single-Crystal Transformation in Microporous Coordination Network {[Ni(cyclam)]3[W(CN)8]2}n. Inorg. Chem. 2007, 46, 8123–8125. [Google Scholar] [CrossRef]
- Nowicka, B.; Reczyński, M.; Bałanda, M.; Fitta, M.; Gaweł, B.; Sieklucka, B. The Rule Rather than the Exception: Structural Flexibility of [Ni(cyclam)]2+-Based Cyano-Bridged Magnetic Networks. Cryst. Growth Des. 2016, 16, 4736–4743. [Google Scholar] [CrossRef]
- Nowicka, B.; Reczyński, M.; Rams, M.; Nitek, W.; Kozieł, M.; Sieklucka, B. Larger pores and higher Tc: {[Ni(cyclam)]3[W(CN)8]2∙solv}n—A new member of the largest family of pseudo-polymorphic isomers among octacyanometallate-based assemblies. CrystEngComm 2015, 17, 3526–3532. [Google Scholar] [CrossRef]
- Nowicka, B.; Heczko, M.; Reczyński, M.; Rams, M.; Gawel, B.; Nitek, W.; Sieklucka, B. Exploration of a new building block for the construction of cyano-bridged solvatomagnetic assemblies: [Ni(cyclam)]3+. CrystEngComm 2016, 18, 7011–7020. [Google Scholar] [CrossRef]
- Nowicka, B.; Reczyński, M.; Rams, M.; Nitek, W.; Zukrowski, J.; Kapusta, C.; Sieklucka, B. Hydration-switchable charge transfer in the first bimetallic assembly based on the [Ni(cyclam)]3+—Magnetic CN-bridged chain {(H3O)[NiIII(cyclam)][FeII(CN)6]∙5H2O}n. Chem. Commun. 2015, 51, 11485–11488. [Google Scholar] [CrossRef]
- Reczyński, M.; Nowicka, B.; Näther, C.; Kozieł, M.; Nakabayashi, K.; Ohkoshi, S.-I.; Sieklucka, B. Dehydration-Triggered Charge Transfer and High Proton Conductivity in (H3O)[NiIII(cyclam)][MII(CN)6] (M = Ru, Os) Cyanide-Bridged Chains. Inorg. Chem. 2018, 57, 13415–13422. [Google Scholar] [CrossRef]
- Colacio, E.; Dominguez-Vera, J.M.; Ghazi, M.; Moreno, J.M.; Kivekas, R.; Lloret, F.; Stoeckli-Evans, H. A novel two-dimensional honeycomb-like bimetallic iron(III)-nickel(II) cyanide-bridged magnetic material [Ni(cyclam)]3[Fe(CN)6]2∙nH2O (cyclam = 1,4,8,11-tetraazacyclodecane). Chem. Commun. 1999, 987–988. [Google Scholar] [CrossRef]
- Ferlay, S.; Mallah, T.; Vaissermann, J.; Bartolome, F.; Veillet, P.; Verdaguer, M. A chromium(III) nickel(II) cyanide-bridged ferromagnetic layered structure with corrugated sheets. Chem. Commun. 1996, 21, 2481–2482. [Google Scholar] [CrossRef]
- Hiroko, T.; Kosuke, N.; Koji, N.; Toshinori, K.; Kazuhito, H.; Shin-ichi, O. Photoreversible Switching of Magnetic Coupling in a Two-dimensional Copper Octacyanomolybdate. Chem. Lett. 2009, 38, 338–339. [Google Scholar] [CrossRef]
- Larionova, J.; Clérac, R.; Donnadieu, B.; Willemin, S.; Guérin, C. Synthesis and Structure of a Two-Dimensional Cyano-Bridged Coordination Polymer [Cu(cyclam)]2[Mo(CN)8]·10.5H2O (Cyclam = 1,4,8,11-Tetraazacyclodecane). Cryst. Growth Des. 2003, 3, 267–272. [Google Scholar] [CrossRef]
- Long, J.; Chamoreau, L.-M.; Marvaud, V. Supramolecular Heterotrimetallic Assembly Based on Octacyanomolybdate, Manganese, and Copper. Eur. J. Inorg. Chem. 2011, 2011, 4545–4549. [Google Scholar] [CrossRef]
- Colacio, E.; Domínguez-Vera, J.M.; Ghazi, M.; Kivekäs, R.; MaríaMoreno, J.; Pajunen, A. Structure and magnetic properties of two cyanide-bridged one-dimensional M–CuII (M = FeIII or FeII) bimetallic assemblies from ferricyanide and CuN42+ (N4 = 1,4,8,11-tetraazacyclotetradecane and N,N ′-bis(2-pyridylmethylene)-1,3-propanediamine) building blocks. J. Chem. Soc. Dalton Trans. 2000, 4, 505–509. [Google Scholar] [CrossRef]
- Salah El Fallah, M.; Ribas, J.; Solans, X.; Font-Bardia, M. A new one-dimensional ferromagnet based on copper(II) and hexacyanochromate(III), with a rope-ladder chain structure. New J. Chem. 2003, 27, 895–898. [Google Scholar] [CrossRef]
- Sen, R.; Bhattacharya, A.; Mal, D.; Bhattacharjee, A.; Gütlich, P.; Mukherjee, A.K.; Solzi, M.; Pernechele, C.; Koner, S. A cyano-bridged bimetallic ferrimagnet: Synthesis, X-ray structure and magnetic study. Polyhedron 2010, 29, 2762–2768. [Google Scholar] [CrossRef]
- Iijima, S.; Honda, Z.; Koner, S.; Mizutani, F. Magnetic and Mössbauer studies of cyanide-bridged bimetallic assembly [Mn(cyclam)][Fe(CN)6]·3H2O. J. Magn. Magn. Mater. 2001, 223, 16–20. [Google Scholar] [CrossRef]
- Bhattacharjee, A.; Miyazaki, Y.; Nakazawa, Y.; Koner, S.; Iijima, S.; Sorai, M. Study of the magnetic phase transition in a cyanide-bridged molecule-based material: [Mn(cyclam)][Fe(CN)6]·3H2O (cyclam=1,4,8,11-tetraazacyclotetradecane). Phys. B Condens. Matter 2001, 305, 56–64. [Google Scholar] [CrossRef]
- Nowicka, B.; Heczko, M.; Rams, M.; Reczyński, M.; Gaweł, B.; Nitek, W.; Sieklucka, B. Solvatomagnetic Studies on Cyano-Bridged Bimetallic Chains Based on [Mn(cyclam)]3+ and Hexacyanometallates. Eur. J. Inorg. Chem. 2017, 1, 99–106. [Google Scholar] [CrossRef]
- Lim, J.H.; You, Y.S.; Yoo, H.S.; Yoon, J.H.; Kim, J.I.; Koh, E.K.; Hong, C.S. Bimetallic MV 2CuII 3 (M = Mo, W) Coordination Complexes Based on Octacyanometalates: Structures and Magnetic Variations Tuned by Chelated Tetradentate Macrocyclic Ligands. Inorg. Chem. 2007, 46, 10578–10586. [Google Scholar] [CrossRef]
- Samotus, A. Photochemical Properties of Octacyanotnagstic Acids. 2. Photolisys of Octacyanotungstic(V) Acid. Pol. J. Chem. 1973, 47, 265–278. [Google Scholar]
- Berry, D.E.; Girard, S.; McAuley, A. The Synthesis and Reactions of Nickel(III) Stabilized by a Nitrogen-Donor Macrocycle. J. Chem. Educ. 1996, 73, 551. [Google Scholar] [CrossRef]
- Sheldrick, G. A short history of SHELX. Acta Crystallogr. A 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Llunell, M.; Casanova, D.; Cirera, J.; Alemany, P.; Alvarez, S. SHAPE v. 2.1; University of Barcelona: Barcelona, Spain, 2013. [Google Scholar]
- Korzeniak, T.; Jankowski, R.; Kozieł, M.; Pinkowicz, D.; Sieklucka, B. Reversible Single-Crystal-to-Single-Crystal Transformation in Photomagnetic Cyanido-Bridged Cd4M2 Octahedral Molecules. Inorg. Chem. 2017, 56, 12914–12919. [Google Scholar] [CrossRef]
- Reczyński, M.; Chorazy, S.; Nowicka, B.; Sieklucka, B.; Ohkoshi, S.-I. Dehydration of Octacyanido-Bridged NiII-WIV Framework toward Negative Thermal Expansion and Magneto-Colorimetric Switching. Inorg. Chem. 2017, 56, 179–185. [Google Scholar] [CrossRef]
- Nowicka, B.; Balanda, M.; Reczyński, M.; Majcher, A.M.; Koziel, M.; Nitek, W.; Lasocha, W.; Sieklucka, B. A water sensitive ferromagnetic [Ni(cyclam)]2[Nb(CN)8] network. Dalton Trans. 2013, 42, 2616–2621. [Google Scholar] [CrossRef]
- Lim, J.H.; Yoon, J.H.; Choi, S.Y.; Ryu, D.W.; Koh, E.K.; Hong, C.S. Cyano-Bridged Pentanuclear and Honeycomblike MIIICuII (M = Fe, Cr) Bimetallic Assemblies: Structural Variations Modulated by Side Groups of Macrocyclic Ligands and Magnetic Properties. Inorg. Chem. 2011, 50, 1749–1757. [Google Scholar] [CrossRef]
- Marvaud, V.; Decroix, C.; Scuiller, A.; Tuyèras, F.; Guyard-Duhayon, C.; Vaissermann, J.; Marrot, J.; Gonnet, F.; Verdaguer, M. Hexacyanometalate Molecular Chemistry: Di-, Tri-, Tetra-, Hexa- and Heptanuclear Heterobimetallic Complexes; Control of Nuclearity and Structural Anisotropy. Chem. Eur. J. 2003, 9, 1692–1705. [Google Scholar] [CrossRef]
- Tuyèras, F.; Scuiller, A.; Duhayon, C.; Hernandez-Molina, M.; de Biani, F.F.; Verdaguer, M.; Mallah, T.; Wernsdorfer, W.; Marvaud, V. Hexacyanidometalate molecular chemistry, part III: Di-, tri-, tetra-, hexa- and hepta-nuclear chromium–nickel complexes: Control of spin, structural anisotropy, intra- and inter-molecular exchange couplings. Inorg. Chim. Acta 2008, 361, 3505–3518. [Google Scholar] [CrossRef]






| 1·5H2O 1 | 1 | 2·3H2O | |
|---|---|---|---|
| Empirical formula | C46H82Cu3N28O5W2 | C46H72Cu3N28W2 | C46H82Cu3N28O5W2 |
| Formula weight | 1665.72 | 1575.57 | 1665.72 |
| Crystal system | triclinic | triclinic | monoclinic |
| Space group | P −1 | P −1 | P 21/n |
| a [Å] | 8.9255(11) | 9.0228(5) | 14.0566(7) |
| b [Å] | 10.0938(13) | 10.8399(8) | 12.8685(7) |
| c [Å] | 19.549(3) | 17.8734(13) | 36.7198(19) |
| α [°] | 78.342(5) | 75.409(6) | 90.0 |
| β [°] | 83.074(5) | 87.810(4) | 100.821(2) |
| γ [°] | 69.472(5) | 60.092(7) | 90.0 |
| V [Å3] | 1613.0(4) | 1458.5(8) | 6524.0(3) |
| Z | 1 | 1 | 4 |
| dc [g·cm−3] | 1.715 | 1.794 | 1.696 |
| µ [mm−1] | 4.590 | 5.064 | 4.539 |
| F(000) | 829 | 779 | 3276 |
| θ range [°] | 1.07–28.28 | 2.79–25.4 | 2.37–29.15 |
| Reflns collected | 21,064 | 5 261 | 17,526 |
| No. of params | 380 | 356 | 746 |
| Rint | 0.0693 | 0.0532 | 0.0362 |
| GooF (F2) | - | 1.018 | 1.059 |
| R1 [I > 2σ(I)] | 0.0693 | 0.0708 | 0.0446 |
| ωR2 (all data) | 0.1662 | 0.1510 | 0.0973 |
| Bond | 1·5H2O 1 | 1 | Angle | 1·5H2O 1 | 1 |
|---|---|---|---|---|---|
| W1-C1 | 2.181 | 2.149 | Cu1-N1-C1 | 143.0 | 129.3 |
| W1-C2 | 2.157 | 2.129 | Cu1-N4-C4 | 157.1 | 135.3 |
| W1-C3 | 2.154 | 2.159 | Cu2-N5-C5 | 157.1 | 142.1 |
| W1-C4 | 2.169 | 2.155 | N11-Cu1-N12 | 93.22 | 86.1 |
| W1-C5 | 2.165 | 2.152 | N12-Cu1-N13 | 86.44 | 95.5 |
| W1-C6 | 2.152 | 2.159 | N13-Cu1-N14 | 94.23 | 86.6 |
| W1-C7 | 2.158 | 2.149 | N14-Cu1-N11 | 85.93 | 91.9 |
| W1-C8 | 2.169 | 2.214 | N21-Cu2-N22 | 86.34 | 86.2 |
| Cu1-N1 | 2.698 | 2.606 | W1-C1-N1 | 176.75 | 176.3 |
| Cu1-N4 | 2.510 | 2.585 | W1-C4-N4 | 177.74 | 179.4 |
| Cu2-N5 | 2.556 | 2.503 | W1-C5-N5 | 174.43 | 176.5 |
| Cu1-N11 | 2.004 | 2.037 | |||
| Cu1-N12 | 2.023 | 2.027 | |||
| Cu1-N13 | 2.024 | 2.018 | |||
| Cu1-N14 | 2.014 | 1.998 | |||
| Cu2-N21 | 2.015 | 2.011 | |||
| Cu2-N22 | 2.032 | 2.028 |
| Cu1-N11 | 2.523 | Cu1-N11-C11 | 157.9 |
| Cu1-O1 | 2.518 | Cu2-N13-C13 | 137.2 |
| Cu2-N13 | 2.596 | Cu3-N21-C21 | 153.9 |
| Cu3-N21 | 2.594 | Cu4-N23-C23 | 142.8 |
| Cu3-O2 | 2.422 | N101-Cu1-N102 | 93.3 |
| Cu4-N23 | 2.457 | N102-Cu1-N103 | 86.0 |
| Cu1-N101 | 2.013 | N103-Cu1-N104 | 95.0 |
| Cu1-N102 | 2.037 | N104-Cu1-N101 | 85.7 |
| Cu1-N103 | 2.011 | N201-Cu2-N202 | 86.0 |
| Cu1-N104 | 2.023 | N301-Cu3-N302 | 85.8 |
| Cu2-N201 | 2.008 | N302-Cu3-N303 | 92.8 |
| Cu2-N202 | 2.019 | N303-Cu3-N304 | 86.4 |
| Cu3-N301 | 2.021 | N304-Cu3-N301 | 95.0 |
| Cu3-N302 | 2.029 | N401-Cu4-N402 | 85.6 |
| Cu3-N303 | 2.008 | W1-C11-N11 | 178.5 |
| Cu3-N304 | 2.013 | W1-C13-N13 | 177.2 |
| Cu4-N401 | 2.024 | W2-C21-N21 | 179.6 |
| Cu4-N402 | 2.016 | W2-C23-N23 | 176.5 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pacanowska, A.; Reczyński, M.; Nowicka, B. Modification of Structure and Magnetic Properties in Coordination Assemblies Based on [Cu(cyclam)]2+ and [W(CN)8]3−. Crystals 2019, 9, 45. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010045
Pacanowska A, Reczyński M, Nowicka B. Modification of Structure and Magnetic Properties in Coordination Assemblies Based on [Cu(cyclam)]2+ and [W(CN)8]3−. Crystals. 2019; 9(1):45. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010045
Chicago/Turabian StylePacanowska, Aleksandra, Mateusz Reczyński, and Beata Nowicka. 2019. "Modification of Structure and Magnetic Properties in Coordination Assemblies Based on [Cu(cyclam)]2+ and [W(CN)8]3−" Crystals 9, no. 1: 45. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9010045