Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Preparation
2.3. Characterization of CaCO3 Particles
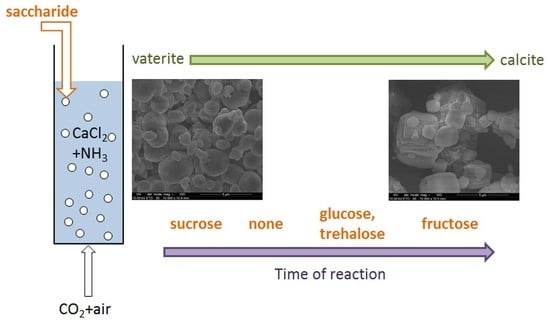
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carr, F.P.; Frederick, D.K. Calcium Carbonate. Kirk-Othmer Encycl. Chem. Technol. 2012, 4, 551–556. [Google Scholar]
- Kitamura, M. Strategy for control of crystallization of polymorphs. Cryst. Eng. Comm. 2009, 11, 949–964. [Google Scholar] [CrossRef]
- Plummer, N.L.; Busenberg, E. The solubilities of calcite, aragonite and vaterite in CO2-H2O solutions between 0 and 90 °C, and an evaluation of the aqueous model for the system CaCO3-CO2-H2O. Geochim. Cosmochim. Acta 1982, 46, 1011–1040. [Google Scholar] [CrossRef]
- Beck, R.; Andreassen, J.P. The onset of spherulitic growth in crystallization of calcium carbonate. J. Cryst. Growth 2010, 312, 2226–2238. [Google Scholar] [CrossRef]
- Rodriguez-Blanco, J.D.; Shaw, S.; Benning, L.G. The kinetics and mechanisms of amorphous calcium carbonate (ACC) crystallization to calcite, via vaterite. Nanoscale 2011, 3, 265–271. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, J.D.; Sand, K.K.; Benning, L.G. ACC and Vaterite as Intermediates in the Solution-Based Crystallization of CaCO3. In New Perspecitves on Mineral Nucleation and Growth; Van Driessche, A.E., Kellermeier, M., Benning, L.G., Gebauer, D., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 93–111. [Google Scholar]
- Trushina, D.B.; Bukreeva, T.V.; Kovalchuk, M.V.; Antipina, M.N. CaCO3 vaterite microparticles for biomedical and personal care applications. Mater. Sci. Eng. C 2014, 45, 644–658. [Google Scholar] [CrossRef] [PubMed]
- Hou, W.; Feng, Q. Morphology and formation mechanism of vaterite particles grown in glycine-containing aqueous solutions. Mater. Sci. Eng. C 2006, 26, 644–647. [Google Scholar] [CrossRef]
- Andreassen, J.-P. Formation mechanism and morphology in precipitation of vaterite-nano-aggregation or crystal growth? J. Cryst. Growth 2005, 274, 256–264. [Google Scholar] [CrossRef]
- Shivkumara, C.; Singh, P.; Gupta, A.; Hegde, M.S. Synthesis of vaterite CaCO3 by direct precipitation using glycine and l-alanine as directing agents. Mater. Res. Bull. 2006, 41, 1455–1460. [Google Scholar] [CrossRef]
- Mori, Y.; Enomae, T.; Isogai, A. Preparation of pure vaterite by simple mechanical mixing of two aqueous salt solutions. Mater. Sci. Eng. C 2009, 29, 1409–1414. [Google Scholar] [CrossRef]
- Trushina, D.B.; Bukreeva, T.V.; Antipina, M.N. Size-Controlled Synthesis of Vaterite Calcium Carbonate by the Mixing Method: Aiming for Nanosized Particles. Cryst. Growth Des. 2016, 16, 1311–1319. [Google Scholar] [CrossRef]
- Han, Y.S.; Hadiko, G.; Fuji, M.; Takahashi, M. Crystallization and transformation of vaterite at controlled pH. J. Cryst. Growth 2006, 289, 269–274. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kościelska, B.; Karczewski, J.; Gołąbiewska, A. The influence of ammonia and selected amines on the characteristics of calcium carbonate precipitated from calcium chloride solutions via carbonation. Mater. Chem. Phys. 2017, 193, 13–18. [Google Scholar] [CrossRef]
- Udrea, I.; Capat, C.; Olaru, E.A.; Isopescu, R.; Mihai, M.; Mateescu, C.D.; Bradu, C. Vaterite synthesis via gas-liquid route under controlled pH conditions. Ind. Eng. Chem. Res. 2012, 51, 8185–8193. [Google Scholar] [CrossRef]
- Dickinson, S.R.; McGrath, K.M. Aqueous precipitation of calcium carbonate modified by hydroxyl-containing compounds. Cryst. Growth Des. 2004, 4, 1411–1418. [Google Scholar] [CrossRef]
- Gómez-Morales, J.; Hernández-Hernández, Á.; Sazaki, G.; García-Ruiz, J.M. Nucleation and polymorphism of calcium carbonate by a vapor diffusion sitting drop crystallization technique. Cryst. Growth Des. 2010, 10, 963–969. [Google Scholar] [CrossRef]
- Prah, J.; Maček, J.; Dražič, G. Precipitation of calcium carbonate from a calcium acetate and ammonium carbamate batch system. J. Cryst. Growth 2011, 324, 229–234. [Google Scholar] [CrossRef]
- Li, Q.; Ding, Y.; Li, F.; Xie, B.; Qian, Y. Solvothermal growth of vaterite in the presence of ethylene glycol, 1,2-propanediol and glycerin. J. Cryst. Growth 2002, 236, 357–362. [Google Scholar] [CrossRef]
- Manoli, F.; Dalas, E. Spontaneous precipitation of calcium carbonate in the presence of ethanol, isopropanol and diethylene glycol. J. Cryst. Growth 2000, 218, 359–364. [Google Scholar] [CrossRef]
- Konopacka-Łyskawa, D.; Kosścielska, B.; Karczewski, J. Effect of some organic solvent-water mixtures composition on precipitated calcium carbonate in carbonation process. J. Cryst. Growth 2015, 418. [Google Scholar] [CrossRef]
- Manoli, F.; Kanakis, J.; Malkaj, P.; Dalas, E. The effect of aminoacids on the crystal growth of calcium carbonate. J. Cryst. Growth 2002, 236, 363–370. [Google Scholar] [CrossRef]
- Chuajiw, W.; Takatori, K.; Igarashi, T.; Hara, H.; Fukushima, Y. The influence of aliphatic amines, diamines, and amino acids on the polymorph of calcium carbonate precipitated by the introduction of carbon dioxide gas into calcium hydroxide aqueous suspensions. J. Cryst. Growth 2014, 386, 119–127. [Google Scholar] [CrossRef]
- Schenk, A.S.; Cantaert, B.; Kim, Y.-Y.; Li, Y.; Read, E.S.; Semsarilar, M.; Armes, S.P.; Meldrum, F.C. Systematic Study of the Effects of Polyamines on Calcium Carbonate Precipitation. Chem. Mater. 2014, 26, 2703–2711. [Google Scholar] [CrossRef]
- Kontrec, J.; Kralj, D.; Brečević, L.; Falini, G. Influence of some polysaccharides on the production of calcium carbonate filler particles. J. Cryst. Growth 2008, 210, 4554–4560. [Google Scholar] [CrossRef]
- Saraya, M.E.-S.I.; Rokbaa, H.H.A.E.-L. Formation and Stabilization of Vaterite Calcium Carbonate by Using Natural Polysaccharide. Adv. Nanopart. 2017, 6, 158–162. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, Y.; Huang, X.; Wu, G. Biomimetic synthesis of calcium carbonate with different morphologies and polymorphs in the presence of bovine serum albumin and soluble starch. Mater. Sci. Eng. C 2017, 79, 457–464. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Berganza, J.A.; Espinosa-Marzal, R.M. Mechanistic Approach to Predict the Combined Effects of Additives and Surface Templates on Calcium Carbonate Mineralization. Cryst. Growth Des. 2016, 16, 6186–6198. [Google Scholar] [CrossRef]
- Yao, C.-L.; Ding, A.-M. Saccharides with Different Molecular Weight Affects Crystallization of Calcium Carbonate. Asian J. Chem. 2013, 25, 2939–2940. [Google Scholar] [CrossRef]
- Polowczyk, I.; Bastrzyk, A.; Fiedot, M. Protein-mediated precipitation of calcium carbonate. Materials 2016, 9, 944. [Google Scholar] [CrossRef] [PubMed]
- Vucak, M.; Peric, J.; Pons, M.-N. The Influence of Various Admixtures on the Calcium Carbonate Precipitation from a Calcium Nitrate and Monoethanolamine Solution. Chem. Eng. Technol. 1998, 21, 71–75. [Google Scholar] [CrossRef]
- García-Carmona, J.; Gómez-Morales, J.; Fraile-Sainz, J.; Rodríguez-Clemente, R. Morphological characteristics and aggregation of calcite crystals obtained by bubbling CO2 through a Ca(OH)2 suspension in the presence of additives. Powder Technol. 2003, 130, 307–315. [Google Scholar] [CrossRef]
- Feng, S.; Bagia, C.; Mpourmpakis, G. Determination of proton affinities and acidity constants of sugars. J. Phys. Chem. A 2013, 112, 5211–5219. [Google Scholar] [CrossRef] [PubMed]
- Urban, F.; Shaffer, P.A. The acidic property of sugars. J. Biol. Chem. 1932, 94, 697–715. [Google Scholar]
- Mani, F.; Peruzzini, M.; Stoppioni, P. CO2 absorption by aqueous NH3 solutions: Speciation of ammonium carbamate, bicarbonate and carbonate by a 13C NMR study. Green Chem. 2006, 8, 995–1000. [Google Scholar] [CrossRef]
- Vázquez, G.; Chenlo, F.; Pereira, G. Enhancement of the Absorption of CO2 in Alkaline Buffers by Organic Solutes: Relation with Degree of Dissociation and Molecular OH Density. Ind. Eng. Chem. Res. 1997, 36, 2353–2358. [Google Scholar] [CrossRef]
- Rao, A.; Berg, J.K.; Kellermeier, M.; Gebauer, D. Sweet on biomineralization: Effects of carbohydrates on the early stages of calcium carbonate crystallization. Eur. J. Mineral. 2014, 26, 537–552. [Google Scholar] [CrossRef]
- Kontoyannis, C.G.; Vagenas, N.V. Calcium carbonate phase analysis using XRD and FT-Raman spectroscopy. Analyst 2000, 125, 251–255. [Google Scholar] [CrossRef]
- Vagenas, N.V.; Gatsouli, A.; Kontoyannis, C.G. Quantitative analysis of synthetic calcium carbonate polymorphs using FT-IR spectroscopy. Talanta 2003, 59, 831–836. [Google Scholar] [CrossRef]
- Kluge, T.; John, C.M. Technical Note: A simple method for vaterite precipitation for isotopic studies: Implications for bulk and clumped isotope analysis. Biogeosciences 2015, 12, 3289–3299. [Google Scholar] [CrossRef]
- Rodriguez-Navarro, C.; Jimenez-Lopez, C.; Rodriguez-Navarro, A.; Gonzalez-Muñoz, M.T.; Rodriguez-Gallego, M. Bacterially mediated mineralization of vaterite. Geochim. Cosmochim. Acta 2007, 71, 1197–1213. [Google Scholar] [CrossRef]
- Kralj, D.; Brecević, L.; Nielsen, A.E. Vaterite growth and dissolution in aqueous solution II. Kinetics of dissolution. J. Cryst. Growth 1994, 143, 269–276. [Google Scholar] [CrossRef]
- Gebauer, D.; Cölfen, H.; Verch, A.; Antonietti, M. The multiple roles of additives in CaCO3 crystallization: A quantitative case study. Adv. Mater. 2009, 21, 435–439. [Google Scholar] [CrossRef]





| Saccharide | µ, mPas1 | pHi | τpH const, min | τr, min |
|---|---|---|---|---|
| - *(C) | 1.11 | 10.9 | 3 | 20 |
| Glucose (G) | 1.27 | 10.4 | 5 | 18 |
| Fructose (F) | 1.41 | 9.8 | 13 | 21 |
| Sucrose (S) | 1.51 | 10.6 | 2 | 8 |
| Trehalose (T) | 1.45 | 10.6 | 6 | 16 |
| Saccharide | XV, % | dV, nm | dC, nm |
|---|---|---|---|
| - (C) | 75 | 220 | 240 |
| Glucose (G) | 56 | 33 | 120 |
| Fructose (F) | 2 | - | 120 |
| Sucrose (S) | 90 | 110 | - |
| Trehalose (T) | 54 | 60 | 72 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Konopacka-Łyskawa, D.; Czaplicka, N.; Kościelska, B.; Łapiński, M.; Gębicki, J. Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method. Crystals 2019, 9, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020117
Konopacka-Łyskawa D, Czaplicka N, Kościelska B, Łapiński M, Gębicki J. Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method. Crystals. 2019; 9(2):117. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020117
Chicago/Turabian StyleKonopacka-Łyskawa, Donata, Natalia Czaplicka, Barbara Kościelska, Marcin Łapiński, and Jacek Gębicki. 2019. "Influence of Selected Saccharides on the Precipitation of Calcium-Vaterite Mixtures by the CO2 Bubbling Method" Crystals 9, no. 2: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9020117