Hybrid Biomimetic Materials from Silica/Carbonate Biomorphs
Abstract
:1. Introduction
2. Materials and Methods
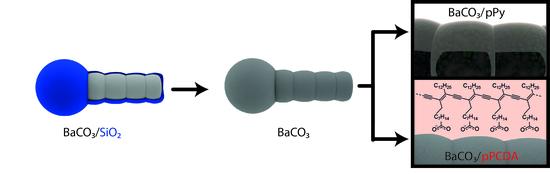
2.1. Biomorph Formation
2.2. pPCDA Functionalization
2.3. pPy Functionalization
2.4. Analytical Methods
3. Results and Discussion
3.1. Preservation with pPCDA
3.2. Preservation with pPy
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kellermeier, M.; Colfen, H.; Garcia-Ruiz, J.M. Silica biomorphs: Complex biomimetic hybrid materials from “sand and chalk”. Eur. J. Inorg. Chem. 2012, 2012, 5123–5144. [Google Scholar] [CrossRef]
- Nakouzi, E.; Steinbock, O. Self-organization in precipitation reactions far from the equilibrium. Sci. Adv. 2016, 2, e1601144. [Google Scholar] [CrossRef]
- Garcia-Ruiz, J.M.; Hyde, S.T.; Carnerup, A.M.; Christy, A.G.; van Kranendonk, M.J.; Welham, N.J. Self-assembled silica-carbonate structures and detection of ancient microfossils. Science 2003, 302, 1194–1197. [Google Scholar] [CrossRef] [PubMed]
- Mann, S. The chemistry of form. Angew. Chem. Int. Ed. 2000, 39, 3392–3406. [Google Scholar] [CrossRef]
- Addadi, L.; Weiner, S. Control and design principles in biological mineralization. Angew. Chem. Int. Ed. 1992, 31, 153–169. [Google Scholar] [CrossRef]
- Lowenstam, H.A.; Weiner, S.; Weiner, S. On Biomineralization; Oxford University Press: New York, NY, USA, 1989; Volume 324. [Google Scholar]
- Kellermeier, M.; Melero-García, E.; Kunz, W.; García-Ruiz, J.M. Local autocatalytic co-precipitation phenomena in self-assembled silica-carbonate materials. J. Colloid Interface Sci. 2012, 380, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Delgado-Lopez, J.M.; Choquesillo-Lazarte, D.; Garcia-Ruiz, J.M. Growth behavior of monohydrocalcite (caco3 center dot h2o) in silica-rich alkaline solution. Cryst. Growth Des. 2015, 15, 564–572. [Google Scholar] [CrossRef]
- Zhang, G.; Garcia-Ruiz, J.M.; Sanchez-Migallon, J.M. Growth behaviour of silica/carbonate nanocrystalline composites of calcite and aragonite. J. Mater. Chem. B 2017, 5, 1658–1663. [Google Scholar] [CrossRef]
- Zhang, G.; Verdugo-Escamilla, C.; Choquesillo-Lazarte, D.; Garcia-Ruiz, J.M. Thermal assisted self-organization of calcium carbonate. Nat. Commun. 2018, 9, 5221. [Google Scholar] [CrossRef]
- Kellermeier, M.; Melero-Garcia, E.; Glaab, F.; Eiblmeier, J.; Kienle, L.; Rachel, R.; Kunz, W.; Garcia-Ruiz, J.M. Growth behavior and kinetics of self-assembled silica-carbonate biomorphs. Chem. Eur. J. 2012, 18, 2272–2282. [Google Scholar] [CrossRef] [PubMed]
- García-Ruiz, J.M.; Melero-García, E.; Hyde, S.T. Morphogenesis of self-assembled nanocrystalline materials of barium carbonate and silica. Science 2009, 323, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Opel, J.; Kellermeier, M.; Sickinger, A.; Morales, J.; Cölfen, H.; García-Ruiz, J.-M. Structural transition of inorganic silica–carbonate composites towards curved lifelike morphologies. Minerals 2018, 8, 75. [Google Scholar] [CrossRef]
- Bittarello, E.; Roberto Massaro, F.; Aquilano, D. The epitaxial role of silica groups in promoting the formation of silica/carbonate biomorphs: A first hypothesis. J. Cryst. Growth 2010, 312, 402–412. [Google Scholar] [CrossRef]
- Opel, J.; Hecht, M.; Rurack, K.; Eiblmeier, J.; Kunz, W.; Colfen, H.; Kellermeier, M. Probing local ph-based precipitation processes in self-assembled silica-carbonate hybrid materials. Nanoscale 2015, 7, 17434–17440. [Google Scholar] [CrossRef]
- Montalti, M.; Zhang, G.; Genovese, D.; Morales, J.; Kellermeier, M.; García-Ruiz, J.M. Local ph oscillations witness autocatalytic self-organization of biomorphic nanostructures. Nat. Commun. 2017, 8, 14427. [Google Scholar] [CrossRef]
- Alexander, G.B.; Heston, W.; Iler, R.K. The solubility of amorphous silica in water. J. Phys. Chem. 1954, 58, 453–455. [Google Scholar] [CrossRef]
- Iler, K.R. The Chemistry of Silica. Solubility, Polymerization, Colloid and Surface Properties and Biochemistry of Silica; John Wiley & Sons: Hoboken, NJ, USA, 1979. [Google Scholar]
- Garcia Ruiz, J.M.; Carnerup, A.; Christy, A.G.; Welham, N.J.; Hyde, S.T. Morphology: An ambiguous indicator of biogenicity. Astrobiology 2002, 2, 353–369. [Google Scholar] [CrossRef] [PubMed]
- Opel, J.; Wimmer, F.P.; Kellermeier, M.; Cölfen, H. Functionalisation of silica–carbonate biomorphs. Nanoscale Horiz. 2016, 1, 144–149. [Google Scholar] [CrossRef]
- Pang, J.B.; Yang, L.; McCaughey, B.F.; Peng, H.S.; Ashbaugh, H.S.; Brinker, C.J.; Lu, Y.F. Thermochromatism and structural evolution of metastable polydiacetylenic crystals. J. Phys. Chem. B 2006, 110, 7221–7225. [Google Scholar] [CrossRef] [PubMed]
- Patlolla, A.; Zunino, J.; Frenkel, A.I.; Iqbal, Z. Thermochromism in polydiacetylene-metal oxide nanocomposites. J. Mater. Chem. 2012, 22, 7028–7035. [Google Scholar] [CrossRef]
- Göppert, A.; Cölfen, H. Infiltration of biomineral templates for nanostructured polypyrrole. Rsc. Adv. 2018, 8, 33748–33752. [Google Scholar] [CrossRef]
- Munekawa, Y.; Oaki, Y.; Imai, H. An experimental study on the processes of hierarchical morphology replication by means of a mesocrystal: A case study of poly (3, 4-ethylenedioxythiophene). Langmuir 2014, 30, 3236–3242. [Google Scholar] [CrossRef]
- Oaki, Y.; Kijima, M.; Imai, H. Synthesis and morphogenesis of organic polymer materials with hierarchical structures in biominerals. J. Am. Chem. Soc. 2011, 133, 8594–8599. [Google Scholar] [CrossRef]
- Janata, J.; Josowicz, M. Conducting polymers in electronic chemical sensors. Nat. Mater. 2003, 2, 19–24. [Google Scholar] [CrossRef] [PubMed]
- Geetha, S.; Rao, C.R.K.; Vijayan, M.; Trivedi, D.C. Biosensing and drug delivery by polypyrrole. Anal. Chim. Acta 2006, 568, 119–125. [Google Scholar] [CrossRef]
- Unni, S.M.; Dhavale, V.M.; Pillai, V.K.; Kurungot, S. High pt utilization electrodes for polymer electrolyte membrane fuel cells by dispersing pt particles formed by a preprecipitation method on carbon “polished” with polypyrrole. J. Phys. Chem. C 2010, 114, 14654–14661. [Google Scholar] [CrossRef]
- Olson, T.S.; Pylypenko, S.; Atanassov, P.; Asazawa, K.; Yamada, K.; Tanaka, H. Anion-exchange membrane fuel cells: Dual-site mechanism of oxygen reduction reaction in alkaline media on cobalt-polypyrrole electrocatalysts. J. Phys. Chem. C 2010, 114, 5049–5059. [Google Scholar] [CrossRef]
- Ge, H.; Wallace, G.G. High-performance liquid chromatography on polypyrrole-modified silica. J. Chromatogr. A 1991, 588, 25–31. [Google Scholar] [CrossRef]
- Choi, C.; Kim, J.H.; Sim, H.J.; Di, J.; Baughman, R.H.; Kim, S.J. Microscopically buckled and macroscopically coiled fibers for ultra-stretchable supercapacitors. Adv. Energy Mater. 2017, 7, 1602021. [Google Scholar] [CrossRef]
- Siglreitmeier, M.; Wu, B.; Kollmann, T.; Neubauer, M.; Nagy, G.; Schwahn, D.; Pipich, V.; Faivre, D.; Zahn, D.; Fery, A.; et al. Multifunctional layered magnetic composites. Beilstein J. Nanotechnol. 2015, 6, 134–148. [Google Scholar] [CrossRef]
- Oaki, Y.; Imai, H. The hierarchical architecture of nacre and its mimetic material. Angew. Chem. Int. Ed. 2005, 44, 6571–6575. [Google Scholar] [CrossRef] [PubMed]
- Oaki, Y.; Imai, H. Nanoengineering in echinoderms: The emergence of morphology from nanobricks. Small 2006, 2, 66–70. [Google Scholar] [CrossRef] [PubMed]
- Dei, S.; Matsumoto, A.; Matsumoto, A. Thermochromism of polydiacetylenes in the solid state and in solution by the self-organization of polymer chains containing no polar group. Macromolecules 2008, 41, 2467–2473. [Google Scholar] [CrossRef]



© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Opel, J.; Unglaube, N.; Wörner, M.; Kellermeier, M.; Cölfen, H.; García-Ruiz, J.-M. Hybrid Biomimetic Materials from Silica/Carbonate Biomorphs. Crystals 2019, 9, 157. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9030157
Opel J, Unglaube N, Wörner M, Kellermeier M, Cölfen H, García-Ruiz J-M. Hybrid Biomimetic Materials from Silica/Carbonate Biomorphs. Crystals. 2019; 9(3):157. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9030157
Chicago/Turabian StyleOpel, Julian, Niklas Unglaube, Melissa Wörner, Matthias Kellermeier, Helmut Cölfen, and Juan-Manuel García-Ruiz. 2019. "Hybrid Biomimetic Materials from Silica/Carbonate Biomorphs" Crystals 9, no. 3: 157. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9030157