Mechanochemical Access to Elusive Metal Diphosphinate Coordination Polymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of [[Cu4(pc2p)4(H2O)6]·8(H2O)]n, 1
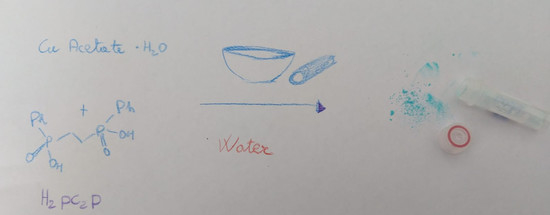
2.2. Synthesis of Cupc2p, 2
2.3. X-Ray Structure Determination
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vioux, A.; Le Bideau, J.; Mutin, P.H.; Leclercq, D. Hybrid Organic-Inorganic Materials Based on Organophosphorus Derivatives. In New Aspects in Phosphorus Chemistry IV. Topics in Current Chemistry; Majoral, J.P., Ed.; Springer: Berlin/Heidelberg, Germany, 2004; Volume 232, pp. 145–174. [Google Scholar]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.; Gaan, S. Recent developments in organophosphorus flame retardants containing P-C bond and their applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef]
- Hynek, J.; Brázda, P.; Rohlíček, J.; Londesborough, M.; Demel, J. Phosphinic Acid Based Linkers: Building Blocks in Metal–Organic Framework Chemistry. Angew. Chem. Int. Ed. 2018, 57, 5016–5019. [Google Scholar] [CrossRef]
- Costantino, F.; Ienco, A.; Taddei, M. Hybrid Multifunctional Materials Based on Phosphonates, Phosphinates and Auxiliary Ligands. In Tailored Organic-Inorganic Materials; Brunet, E., Colon, J.L., Clearfield, A., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 193–244. [Google Scholar]
- Costantino, F.; Ienco, A.; Taddei, M. The Influence of Non-covalent Interactions in the Structure and Dimensionality of Hybrid Compounds and Coordination Polymers in Non-Covalent Interactions. In Synthesis and Design of New Compounds; Maharramov, A.M., Mahmudov, K.T., Kopylovich, M.N., Pombeiro, A.J.L., Eds.; John Wiley & Sons: Hoboken, NJ, USA, 2016; pp. 163–184. [Google Scholar]
- David, T.; Procházková, S.; Kotek, J.; Kubíček, V.; Hermann, P.; Lukeš, I. Aminoalkyl-1,1-bis(phosphinic acids): Stability, acid-base, and coordination properties. Eur. J. Inorg. Chem. 2014, 2014, 4357–4368. [Google Scholar] [CrossRef]
- David, T.; Procházková, S.; Havlíčková, J.; Kotek, J.; Kubíček, V.; Hermann, P.; Lukeš, I. Methylene-bis[(aminomethyl)phosphinic acids]: Synthesis, acid-base and coordination properties. Dalton Trans. 2013, 42, 2414–2422. [Google Scholar] [CrossRef] [PubMed]
- Cecconi, F.; Dominguez, S.; Masciocchi, N.; Midollini, S.; Sironi, A.; Vacca, A. Complexation of beryllium(II)ion by phosphinate ligands in aqueous solution. Synthesis and XRPD structure determination of Be[(PhPO2)2CH2](H2O)2. Inorg. Chem. 2003, 42, 2350–2356. [Google Scholar] [CrossRef]
- Midollini, S.; Lorenzo-Luis, P.; Orlandini, A. Inorganic-organic hybrid materials of p,p′-diphenylmethylenediphosphinic acid (H2pcp) with magnesium and calcium ions: Synthesis and characterization of [Mg(Hpcp)2], [Mg(Hpcp)2(H2O)4], [Mg(pcp)(H2O)3](H2O), [Ca(Hpcp)2] and [Ca(pcp)(H2O)] complexes. Inorg. Chim. Acta 2006, 359, 3275–3282. [Google Scholar] [CrossRef]
- Costantino, F.; Gentili, P.; Guerri, A.; Ienco, A.; Midollini, S.; Oberhauser, W. Structural similarities in 1D coordination polymers of alkaline earth diphosphinates. Inorg. Chim. Acta 2012, 391, 150–157. [Google Scholar]
- Beckmann, J.; Costantino, F.; Dakternieks, D.; Duthie, A.; Ienco, A.; Midollini, S.; Mitchell, C.; Orlandini, A.; Sorace, L. Inorganic-organic hybrids of the p,p′-diphenylmethylenediphosphinate, pcp2−. Synthesis, characterization, and XRPD structures of [Sn(pcp)] and [Cu(pcp)]. Inorg. Chem. 2005, 44, 9416–9423. [Google Scholar]
- Cecconi, F.; Ghilardi, C.; Midollini, S.; Orlandini, A. A new lead(II) inorganic-organic hybrid of the P,P′-diphenylmethylene-diphosphinate ligand: Synthesis and X-ray characterization of the [Pb(CH2(P(Ph)O2)2)] complex. Inorg. Chem. Commun. 2003, 6, 546–548. [Google Scholar] [CrossRef]
- Lyssenko, K.A.; Vologzhanina, A.V.; Torubaev, Y.V.; Nelyubina, Y.V. A comparative study of a mixed-ligand Copper(II) complex by the theory of atoms in molecules and the Voronoi tessellation. Mendeleev Commun. 2014, 24, 216–218. [Google Scholar] [CrossRef]
- Ciattini, S.; Costantino, F.; Lorenzo-Luis, P.; Midollini, S.; Orlandini, A.; Vacca, A. Inorganic-organic hybrids formed by p,p′- diphenylmethylenediphosphinate, pcp2−, with the Cu2+ ion. x-ray crystal structures of [Cu(pcp)H2O2]·H2O and [Cu(pcp)bipy(H2O)]. Inorg. Chem. 2005, 44, 4008–4016. [Google Scholar] [CrossRef]
- Cecconi, F.; Dakternieks, D.; Duthie, A.; Ghilardi, C.; Gili, P.; Lorenzo-Luis, P.; Midollini, S.; Orlandini, A. Inorganic-organic hybrids of the p,p′-diphenylmethylenediphosphinate ligand with bivalent metals: A new 2D-layered phenylphosphinate zinc(II) complex. J. Solid State Chem. 2004, 177, 786–792. [Google Scholar] [CrossRef]
- Berti, E.; Cecconi, F.; Ghilardi, C.; Midollini, S.; Orlandini, A.; Pitzalis, E. Isostructural organic-inorganic hybrids of P,P′-diphenyl-methylenediphosphinate (CH2(P(Ph)O2)2)2− with divalent transition metals. Inorg. Chem. Commun. 2002, 5, 1041–1043. [Google Scholar]
- Costantino, F.; Midollini, S.; Orlandini, A.; Sorace, L. Hydrothermal synthesis and structural characterization of a new 2D-layered vanadium diphosphinate: [VO(O2(C6H5)PCH2P(C6H5)O2)]. Inorg. Chem. Commun. 2006, 9, 591–594. [Google Scholar] [CrossRef]
- Midollini, S.; Orlandini, A. Hydrogen bonding in triamine copper(II) P,P′- Diphenyl methylene diphosphinate (pcp2−) hybrids. Syntheses and crystal structures of [Cu(pcp)(2,2′-dipyridylamine)(H2O)]·2H2O and [Cu(pcp)(2,2′:6′,2″terpyridine)]·4 H2O. J. Coord. Chem. 2006, 59, 1433–1442. [Google Scholar] [CrossRef]
- Ienco, A.; Midollini, S.; Orlandini, A.; Costantino, F. Synthesis and structural characterization of a tetranuclear zinc(II) complex with P,P′-diphenylmethylenediphosphinate (pcp) and 2,2′-bipyridine (2,2′-bipy) ligands. Z. Naturforsch. B Chem. Sci. 2007, 62, 1476–1480. [Google Scholar] [CrossRef]
- Costantino, F.; Ienco, A.; Midollini, S.; Orlandini, A.; Sorace, L.; Vacca, A. Copper(II) complexes with bridging diphosphinates—The effect of the elongation of the aliphatic chain on the structural arrangements around the metal centres. Eur. J. Inorg. Chem. 2008, 2008, 3046–3055. [Google Scholar] [CrossRef]
- Taddei, M.; Ienco, A.; Costantino, F.; Guerri, A. Supramolecular interactions impacting on the water stability of tubular metal-organic frameworks. RSC Adv. 2013, 3, 26177–26183. [Google Scholar] [CrossRef]
- Ienco, A.; Caporali, M.; Costantino, F.; Guerri, A.; Manca, G.; Moneti, S.; Peruzzini, M. The quest for hydrogen bond-based metal organic nanotubes (MONT). J. Coord. Chem. 2014, 67, 3863–3872. [Google Scholar] [CrossRef]
- Costantino, F.; Midollini, S.; Orlandini, A. Cobalt(II) and nickel(II) coordination polymers constructed from P,P′-diphenylmethylenediphosphinic acid (H2pcp) and 4,4′-bipyridine (bipy): Structural isomerism in [Co(pcp)(bipy)0.5(H2O)2]. Inorg. Chim. Acta 2008, 361, 327–334. [Google Scholar] [CrossRef]
- Bataille, T.; Costantino, F.; Lorenzo-Luis, P.; Midollini, S.; Orlandini, A. A new copper(II) tubelike metal-organic framework constructed from P,P′-diphenylmethylenediphosphinic acid and 4,4′-bipyridine: Synthesis, structure, and thermal behavior. Inorg. Chim. Acta 2008, 361, 9–15. [Google Scholar] [CrossRef]
- Bataille, T.; Bracco, S.; Comotti, A.; Costantino, F.; Guerri, A.; Ienco, A.; Marmottini, F. Solvent dependent synthesis of micro-and nano-crystalline phosphinate based 1D tubular MOF: Structure and CO2 adsorption selectivity. CrystEngComm 2012, 14, 7170–7173. [Google Scholar] [CrossRef]
- Bataille, T.; Costantino, F.; Ienco, A.; Guerri, A.; Marmottini, F.; Midollini, S. A snapshot of a coordination polymer self-assembly process: The crystallization of a metastable 3D network followed by the spontaneous transformation in water to a 2D pseudopolymorphic phase. Chem. Commun. 2008, 2008, 6381–6383. [Google Scholar] [CrossRef] [PubMed]
- Costantino, F.; Ienco, A.; Midollini, S. Different structural networks determined by variation of the ligand skeleton in copper(II) diphosphinate coordination polymers. Cryst. Growth Des. 2010, 10, 7–10. [Google Scholar] [CrossRef]
- Guerri, A.; Taddei, M.; Bataille, T.; Moneti, S.; Boulon, M.; Sangregorio, C.; Costantino, F.; Ienco, A. Same Not the Same: Thermally Driven Transformation of Nickel Phosphinate-Bipyridine One-Dimensional Chains into Three-Dimensional Coordination Polymers. Cryst. Growth Des. 2018, 18, 2234–2242. [Google Scholar] [CrossRef] [Green Version]
- Bowmaker, G.A. Solvent-assisted mechanochemistry. Chem. Commun. 2013, 49, 334–348. [Google Scholar]
- Alexandrov, E.V.; Shevchenko, A.; Blatov, V.A. Topological databases: Why do we need them for design of coordination polymers? Cryst. Growth Des. 2019. [Google Scholar] [CrossRef]
- Garst, M.E. Alkylation of Phenyl Phosphinic Acid. Synth. Commun. 1979, 9, 261–266. [Google Scholar] [CrossRef]
- CrysAlisCCD; Version1.171.33.41 (release06-05-2009 CrysAlis171.NET); Oxford Diffraction Ltd.: Abingdon, UK, 2009.
- CrysAlisRED; Version1.171.33.41 (release06-05-2009 CrysAlis171.NET); Oxford Diffraction Ltd.: Abingdon, UK, 2009.
- Altomare, A.; Burla, M.C.; Camalli, M.; Cascarano, G.L.; Giacovazzo, C.; Guagliardi, A.; Moliterni, A.G.G.; Polidori, G.; Spagna, R. SIR97: A new tool for crystal structure determination and refinement. J. Appl. Crystallogr. 1999, 32, 115–119. [Google Scholar] [CrossRef]
- Sheldrick, G.M. A short history of SHELX. Acta Crystallogr. Sect. A Found. Crystallogr. 2008, 64, 112–122. [Google Scholar] [CrossRef]
- Bacci, M. Jahn-Teller effect in five-coordinated copper(II) complexes. Chem. Phys. Lett. 1986, 104, 191–199. [Google Scholar] [CrossRef]
- Bonneau, C.; O’Keeffe, M.; Proserpio, D.; Blatov, V.; Batten, S.; Bourne, S.; Lah, M.; Eon, J.; Hyde, S.; Wiggin, S.; et al. Deconstruction of Crystalline Networks into Underlying Nets: Relevance for Terminology Guidelines and Crystallographic Databases. Cryst. Growth Des. 2018, 18, 3411–3418. [Google Scholar] [CrossRef]
- Blatov, V.A.; Shevchenko, A.P.; Proserpio, D.M. Applied Topological Analysis of Crystal Structures with the Program Package ToposPro. Cryst. Growth Des. 2014, 14, 3576–3586. [Google Scholar] [CrossRef]
- Alexandrov, E.V.; Blatov, V.A.; Kochetkov, A.V.; Proserpio, D.M. Underlying nets in three-periodic coordination polymers: Topology, taxonomy and prediction from a computer-aided analysis of the Cambridge Structural Database. CrystEngComm 2011, 13, 3947–3958. [Google Scholar] [CrossRef]
- Mitina, T.G.; Blatov V., A. Topology of 2-Periodic Coordination Networks: Toward Expert Systems in Crystal Design. Cryst. Growth Des. 2013, 13, 1655–1664. [Google Scholar] [CrossRef]
- TOPCryst Topological Database. Available online: https://topcryst.com/ (accessed on 14 April 2019).
- Sachdeva, N.; Dolzhenko, A.; Keung Chui, W. Regioselective synthesis of pyrimido[1,2-a][1,3,5]triazin-6-ones via reaction of 1-(6-oxo-1,6-dihydropyrimidin-2-yl)guanidines with triethylorthoacetate: Observation of an unexpected rearrangement. Org. Biomol. Chem. 2012, 10, 4586–4596. [Google Scholar] [CrossRef]
- Wu, Y.-J.; He, H.; Sun, L.-Q.; Wu, D.; Gao, Q.; Li, H.-Y. Synthesis of fluorinated 1-(3-Morpholin-4-yl-phenyl)-Ethylamines. Bioorg. Med. Chem. Lett. 2003, 13, 1725–1728. [Google Scholar] [CrossRef]
- Li, C.; An, C.; Li, X.; Gao, S.; Cui, C.; Sun, H.; Wang, B. Triazole and Dihydroimidazole Alkaloids from the Marine Sediment-Derived Fungus Penicillium paneum SD-44. J. Nat. Prod. 2011, 74, 1331–1334. [Google Scholar] [CrossRef]
- Groom, C.R.; Bruno, I.J.; Lightfoot, M.P.; Ward, S.C. The Cambridge Structural Database. Acta Cryst. 2016, B72, 171–179. [Google Scholar] [CrossRef]






| Empirical formula | C56 H84 Cu4 O30 P8 |
| Formula weight | 1739.15 |
| Temperature | 153(2) K |
| Wavelength | 1.5418 Å |
| Crystal system | Monoclinic |
| Space group | P 2/c |
| Unit cell dimensions | a = 10.4000(10) Å |
| b = 10.355(2) Å | |
| c = 33.277(2) Å | |
| β = 99.033(8)° | |
| Volume | 3539.2(8) Å3 |
| Z | 2 |
| Density (calculated) | 1.632 Mg/m3 |
| Absorption coefficient | 3.793 mm−1 |
| F(000) | 1792 |
| Crystal size | 0.16 × 0.15 × 0.12 mm3 |
| Theta range for data collection | 4.269 to 72.397° |
| Index ranges | −12 < = h< = 12, −12 <= k<= 12, −41 <= l < = 34 |
| Reflections collected | 55473 |
| Independent reflections | 6660 [Rint = 0.0430] |
| Completeness to θ = 26.06° | 99.6% |
| Refinement method | Full-matrix least-squares on F2 |
| Data/restraints/parameters | 6660/11/484 |
| Goodness-of-fit on F2 | 1.067 |
| Final R indices [I > 2σ (I)] | R1 = 0.0287, wR2 = 0.0809 |
| R indices (all data) | R1 = 0.0337, wR2 = 0.0847 |
| Largest diff. peak and hole | 0.451 and −0.621 e.Å−3 |
| Cu1-O1 | 1.9221(15) | Cu3-O31 | 1.9790(15) | O3#2-Cu1-O7#1 | 88.89(6) |
| Cu1-O5 | 1.9258(14) | P2-O4 | 1.5115(16) | O8 -Cu2-O8#3 | 145.44(9) |
| Cu1-O3#2 | 1.9663(15) | P3-O5 | 1.5141(15) | O8-Cu2-O20 | 107.28(5) |
| Cu1-O7#1 | 1.9313(14) | P3-O6 | 1.5088(15) | O8-Cu2-O21 | 86.82(6) |
| Cu1-O20 | 2.4651(14) | P4-O7 | 1.5107(15) | O8#3-Cu2-O21 | 93.49(6) |
| Cu2-O8 | 1.9235(14) | P4-O8 | 1.5087(15) | O21-Cu2-O20 | 89.47(5) |
| Cu2-O20 | 2.203(2) | - | - | O21-Cu2-O21#3 | 178.95(9) |
| Cu2-O21 | 1.9938(15) | O6-Cu3-O6#4 | 179.06(9) | ||
| Cu3-O6 | 1.9293(14) | O1-Cu1-O3#2 | 178.36(7) | O6-Cu3-O30 | 90.47(4) |
| Cu3-O30 | 2.231(3) | O1-Cu1-O5 | 89.84(6) | O31-Cu3-O30 | 92.33(5) |
| P1-O1 | 1.5115(15) | O1-Cu1-O7#1 | 90.52(6) | O6-Cu3-O31 | 87.52(6) |
| P1-O2 | 1.5121(16) | O5-Cu1-O3#2 | 90.95(6) | O6-Cu3-O31#4 | 92.44(6) |
| P2-O3 | 1.5330(15) | O5-Cu1-O7#1 | 172.03(6) | O31-Cu3-O31#4 | 175.33(9) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ienco, A.; Tuci, G.; Guerri, A.; Costantino, F. Mechanochemical Access to Elusive Metal Diphosphinate Coordination Polymer. Crystals 2019, 9, 283. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060283
Ienco A, Tuci G, Guerri A, Costantino F. Mechanochemical Access to Elusive Metal Diphosphinate Coordination Polymer. Crystals. 2019; 9(6):283. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060283
Chicago/Turabian StyleIenco, Andrea, Giulia Tuci, Annalisa Guerri, and Ferdinando Costantino. 2019. "Mechanochemical Access to Elusive Metal Diphosphinate Coordination Polymer" Crystals 9, no. 6: 283. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060283