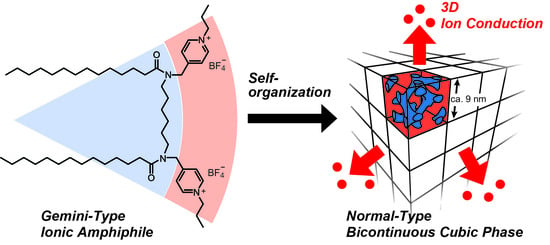
Design of Ionic Liquid Crystals Forming Normal-Type Bicontinuous Cubic Phases with a 3D Continuous Ion Conductive Pathway
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
Thermotropic Liquid–Crystalline Properties
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Ohno, H. Functional Design of Ionic liquids. Bull. Chem. Soc. Jpn. 2006, 79, 1665–1680. [Google Scholar] [CrossRef]
- Ohno, H.; Yoshizawa-Fujita, M.; Kohno, Y. Functional Design of Ionic Liquids Unprecedented Liquids that Contribute to Energy Technology, Bioscience, and Materials Sciences. Bull. Chem. Soc. Jpn. 2019, 92, 852–868. [Google Scholar] [CrossRef]
- MacFarlane, D.R.; Forsyth, M.; Howlett, P.C.; Kar, M.; Passerini, S.; Pringle, J.M.; Ohno, H.; Watanabe, M.; Yan, F.; Zheng, W.; et al. Ionic liquids and their solid-state analogues as materials for energy generation and storage. Nat. Rev. Mater. 2016, 1, 15005. [Google Scholar] [CrossRef]
- Navarra, M.A.; Fujimura, K.; Sgambetterra, M.; Tsurumaki, A.; Panero, S.; Nakamura, N.; Ohno, H.; Scrosati, B. New Ether-functionalized Morpholinium and Piperidinium-based Ionic Liquids as Electrolyte Components in Lithium and Lithium-Ion Batteries. ChemSusChem 2017, 10, 2496–2504. [Google Scholar] [CrossRef] [PubMed]
- Grätzel, M. Dye-sensitized solar cells. J. Photochem. Photobiol. Photochem. Rev. 2003, 4, 145–153. [Google Scholar] [CrossRef]
- Yasuda, T.; Watanabe, M. Protic ionic liquids: Fuel cell applications. MRS Bull. 2013, 38, 560–566. [Google Scholar] [CrossRef]
- Galinski, M.; Lewandowski, A.; Stepniak, I. Ionic liquids as electrolytes. Electrochim. Acta 2006, 51, 5567–5580. [Google Scholar] [CrossRef]
- Kato, T.; Yoshio, M.; Ichikawa, T.; Soberats, B.; Ohno, H.; Funahashi, M. Transport of ions and electrons in nanostructured liquid crystals. Nat. Rev. Mater. 2017, 2, 17001. [Google Scholar] [CrossRef]
- Yoshio, M.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion Transport in Self-Organized Columnar Ionic Liquids. J. Am. Chem. Soc. 2004, 126, 994–995. [Google Scholar] [CrossRef]
- Yoshio, M.; Kagata, T.; Hoshino, K.; Mukai, T.; Ohno, H.; Kato, T. One-Dimensional Ion-Conductive Polymer Films: Alignment and Fixation of Ionic Channels Formed by Self-Organization of Polymerizable Columnar Liquid Crystals. J. Am. Chem. Soc. 2006, 128, 5570–5577. [Google Scholar] [CrossRef]
- Yoshio, M.; Mukai, T.; Kanie, K.; Yoshizawa, M.; Ohno, H.; Kato, T. Layered Ionic Liquids: Anisotropic Ion Conduction in New Self-Organized Liquid-Crystalline Materials. Adv. Mater. 2002, 14, 351–354. [Google Scholar] [CrossRef]
- Sakuda, J.; Yoshio, M.; Ichikawa, T.; Ohno, H.; Kato, T. 2D assemblies of ionic liquid crystals based on imidazolium moieties: Formation of ion-conductive layers. New J. Chem. 2015, 39, 4471–4477. [Google Scholar] [CrossRef]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Mukai, T.; Ohno, H.; Kato, T. Self-Organization of Room-Temperature Ionic Liquids Exhibiting Liquid-Crystalline Bicontinuous Cubic Phases: Formation of Nano-Ion Channel Networks. J. Am. Chem. Soc. 2007, 129, 10662–10663. [Google Scholar] [CrossRef] [PubMed]
- Ichikawa, T.; Yoshio, M.; Hamasaki, A.; Taguchi, S.; Liu, F.; Zeng, X.B.; Ungar, G.; Ohno, H.; Kato, T. Induction of Thermotropic Bicontinuous Cubic Phases in Liquid-Crystalline Ammonium and Phosphonium Salts. J. Am. Chem. Soc. 2012, 134, 2634–2643. [Google Scholar] [CrossRef] [PubMed]
- Goossens, K.; Lava, K.; Bielawski, C.W.; Binnemans, K. Ionic Liquid Crystals: Versatile Materials. Chem. Rev. 2016, 116, 4643–4807. [Google Scholar] [CrossRef] [PubMed]
- Axenov, K.V.; Laschat, S. Thermotropic Ionic Liquid Crystals. Materials 2011, 4, 206–259. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Slattery, J.M.; Bruce, D.W. Columnar thermotropic mesophases formed by dimeric liquid-crystalline ionic liquids exhibiting large mesophase ranges. New J. Chem. 2011, 35, 2910–2918. [Google Scholar] [CrossRef]
- Park, G.; Goossens, K.; Shin, T.J.; Bielawski, C.W. Dicyanamide Salts that Adopt Smectic, Columnar, or Bicontinuous Cubic Liquid-Crystalline Mesophases. Chem. Eur. J. 2018, 24, 6399–6411. [Google Scholar] [CrossRef] [PubMed]
- Goodby, J.W.; Collings, P.J.; Kato, T.; Tschierske, C.; Gleeson, H.; Raynes, P. Handbook of Liquid Crystals, 2nd ed.; Wiley-VCH: Weinheim, Germany, 2014. [Google Scholar]
- Kato, T.; Mizoshita, N.; Kishimoto, K. Functional liquid-crystalline assemblies: Self-organized soft materials. Angew. Chem. Int. Ed. 2006, 45, 38–68. [Google Scholar] [CrossRef] [PubMed]
- Tschierske, C. Non-conventional liquid crystals—The importance of micro-segregation for self-organisation. J. Mater. Chem. 1998, 8, 1485–1508. [Google Scholar] [CrossRef]
- Tschierske, C. Liquid crystal engineering-new complex mesophase structures and their relations to polymer morphologies, nanoscale patterning and crystal engineering. Chem. Soc. Rev. 2007, 36, 1930–1970. [Google Scholar] [CrossRef] [PubMed]
- Diele, S. On thermotropic cubic mesophases. Curr. Opin. Colloid Interface Sci. 2002, 7, 333–342. [Google Scholar] [CrossRef]
- Impéror-Clerc, M. Thermotropic cubic mesophases. Curr. Opin. Colloid Interface Sci. 2005, 9, 370–376. [Google Scholar] [CrossRef]
- Bruce, D.W. Calamitics, Cubics, and Columnars Liquid-Crystalline Complexes of Silver(I). Acc. Chem. Res. 2000, 33, 831–840. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Nemade, P.R.; Lu, X.; Zeng, X.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. New Type of Membrane Material for Water Desalination Based on a Cross-Linked Bicontinuous Cubic Lyotropic Liquid Crystal Assembly. J. Am. Chem. Soc. 2007, 129, 9574–9575. [Google Scholar] [CrossRef] [PubMed]
- Hatakeyama, E.S.; Wiesenauer, B.R.; Gabriel, C.J.; Noble, R.D.; Gin, D.L. Nanoporous, Bicontinuous Cubic Lyotropic Liquid Crystal Networks via Polymerizable Gemini Ammonium Surfactants. Chem. Mater. 2010, 22, 4525–4527. [Google Scholar]
- Sorenson, G.P.; Coppage, K.L.; Mahanthappa, M.K. Unusually Stable Aqueous Lyotropic Gyroid Phases from Gemini Dicarboxylate Surfactants. J. Am. Chem. Soc. 2011, 133, 14928–14931. [Google Scholar] [CrossRef]
- Sorensona, G.P.; Mahanthappa, M.K. Unexpected role of linker position on ammonium gemini surfactant lyotropic gyroid phase stability. Soft Mater. 2016, 12, 2408–2415. [Google Scholar] [CrossRef]
- Matsumoto, T.; Ono, A.; Ichikawa, T.; Kato, T.; Ohno, H. Construction of gyroid-structured matrices through the design of geminized amphiphilic zwitterions and their self-organization. Chem. Commun. 2016, 52, 12167–12170. [Google Scholar] [CrossRef]
- Ono, A.; Ohno, H.; Kato, T.; Ichikawa, T. Design of 3D continuous proton conduction pathway by controlling co-organization behavior of gemini amphiphilic zwitterions and acids. Solid State Ion. 2018, 317, 39–45. [Google Scholar] [CrossRef]
- Hayes, R.; Warr, G.G.; Atkin, R. Structure and Nanostructure in Ionic Liquids. Chem. Rev. 2015, 115, 6357–6426. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ichikawa, T.; Kato, T.; Ohno, H. 3D Continuous Water Nanosheet as a Gyroid Minimal Surface Formed by Bicontinuous Cubic Liquid-Crystalline Zwitterions. J. Am. Chem. Soc. 2012, 134, 11354–11357. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ichikawa, T.; Kato, T.; Ohno, H. Development of Glassy Bicontinuous Cubic Liquid Crystals for Solid Proton-Conductive Materials. Adv. Mater. 2017, 29, 1604429. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, T.; Ichikawa, T. Design of Viologen-Based Liquid Crystals Exhibiting Bicontinuous Cubic Phases and Their Redox-Active Behavior. Materials 2017, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Neve, F.; Impéror-Clerc, M. An Ia3d thermotropic cubic phase from N-alkylpyridinium tetrahalocuprates. Liq. Cryst. 2004, 31, 907–912. [Google Scholar] [CrossRef]
- Kobayashi, T.; Li, Y.X.; Ono, A.; Zeng, Z.B. Ichikawa, Gyroid structured aqua-sheets with sub-nanometer thickness enabling 3D fast proton relay conduction. Chem. Sci. 2019. [Google Scholar] [CrossRef]
- Noda, A.; Watanabe, M. Highly conductive polymer electrolytes prepared by in situ polymerization of vinyl monomers in room temperature molten salts. Electrochim. Acta 2000, 45, 1265–1270. [Google Scholar] [CrossRef]
- Cai, M.; Liang, Y.; Zhou, F.; Liu, W. Functional ionic gels formed by supramolecular assembly of a novel low molecular weight anticorrosive/antioxidative gelator. J. Mater. Chem. 2011, 21, 13399–13405. [Google Scholar] [CrossRef]
- Swatloski, R.P.; Spear, S.K.; Holbrey, J.D.; Rogers, R.D. Dissolution of Cellose with Ionic Liquids. J. Am. Chem. Soc. 2002, 124, 4974–4975. [Google Scholar] [CrossRef]
- Fukaya, Y.; Hayashi, K.; Wada, M.; Ohno, H. Cellulose dissolution with polar ionic liquids under mild conditions: Required factors for anions. Green Chem. 2008, 10, 44–46. [Google Scholar] [CrossRef]
- Bates, E.D.; Mayton, R.D.; Ntai, I.; Davis, J.H., Jr. CO2 Capture by a Task-Specific Ionic Liquid. J. Am. Chem. Soc. 2002, 124, 926–927. [Google Scholar] [CrossRef] [PubMed]
- Mittenthal, M.S.; Flowers, B.S.; Bara, J.E.; Whitley, J.W.; Spear, S.K.; Roveda, J.D.; Wallace, D.A.; Shannon, M.S.; Holler, R.; Martens, R.; et al. Ionic Polyimides: Hybrid Polymer Architectures and Composites with Ionic Liquids for Advanced Gas Separation Membranes. Ind. Eng. Chem. Res. 2017, 56, 5055–5069. [Google Scholar] [CrossRef]
- Adawiyah, N.; Moniruzzaman, M.; Hawatulaila, S.; Goto, M. Ionic liquids as a potential tool for drug delivery systems. Med. Chem. Commun. 2016, 7, 1881–1897. [Google Scholar] [CrossRef]






| Compound | Phase Transition Behavior a) | ||||
|---|---|---|---|---|---|
| 1 | Iso | 38 (-) | Cubbi | 12 (–14) | Cr |
| 2 | Iso | –8 (–20) | Cr | ||
| 3 | Iso | 109 (–0.4) | SmA | 33 (–39) | Cr’ |
| 4 | Iso | –3 (–27) | Cr | ||
| 5 | Iso | 10 (–38) | Cr | ||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ichikawa, T.; Sasaki, Y.; Kobayashi, T.; Oshiro, H.; Ono, A.; Ohno, H. Design of Ionic Liquid Crystals Forming Normal-Type Bicontinuous Cubic Phases with a 3D Continuous Ion Conductive Pathway. Crystals 2019, 9, 309. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060309
Ichikawa T, Sasaki Y, Kobayashi T, Oshiro H, Ono A, Ohno H. Design of Ionic Liquid Crystals Forming Normal-Type Bicontinuous Cubic Phases with a 3D Continuous Ion Conductive Pathway. Crystals. 2019; 9(6):309. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060309
Chicago/Turabian StyleIchikawa, Takahiro, Yui Sasaki, Tsubasa Kobayashi, Hikaru Oshiro, Ayaka Ono, and Hiroyuki Ohno. 2019. "Design of Ionic Liquid Crystals Forming Normal-Type Bicontinuous Cubic Phases with a 3D Continuous Ion Conductive Pathway" Crystals 9, no. 6: 309. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9060309