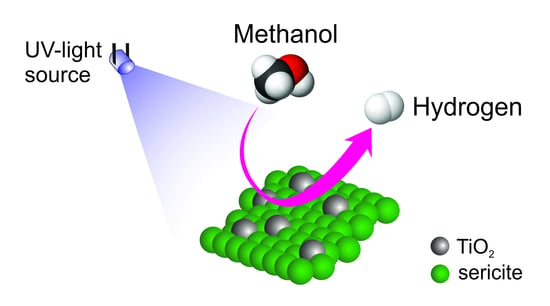
Preparation and Characterization of Nanocrystalline TiO2 on Microsericite for High-Efficiency Photo-Energy Conversion of Methanol to Hydrogen
Abstract
:1. Introduction
2. Materials and Methods
3. Results
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- García-Borboroglu, P.; Boersma, P.D.; Ruoppolo, V.; Reyes, L.; Rebstock, G.A.; Griot, K.; Heredia, S.R.; Adornes, A.C.; Da Silva, R.P. Chronic oil pollution harms Magellanic penguins in the Southwest Atlantic. Mar. Pollut. Bull. 2006, 52, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Bosco, M.; Varrica, D.; Dongarra, G. Case study: Inorganic pollutants associated with particulate matter from an area near a petrochemical plant. Environ. Res. 2005, 99, 18–30. [Google Scholar] [CrossRef] [PubMed]
- Haryanto, A.; Fernando, S.; Murali, N.; Adhikari, S. Current status of hydrogen production techniques by steam reforming of ethanol: A review. Energy Fuels 2005, 19, 2098–2106. [Google Scholar] [CrossRef]
- Palo, D.R.; Dagle, R.A.; Holladay, J.D. Methanol steam reforming for hydrogen production. Chem. Rev. 2007, 107, 3992–4021. [Google Scholar] [CrossRef] [PubMed]
- Funk, J.E. Thermochemical hydrogen production: Past and present. Int. J. Hydrogen. Energ 2001, 26, 185–190. [Google Scholar] [CrossRef]
- Zeng, K.; Zhang, D. Recent progress in alkaline water electrolysis for hydrogen production and applications. Prog. Energy Combust. Sci. 2010, 36, 307–326. [Google Scholar] [CrossRef]
- Holladay, J.D.; Hu, J.; King, D.L.; Wang, Y. An overview of hydrogen production technologies. Catal. Today 2009, 139, 244–260. [Google Scholar] [CrossRef]
- Liao, C.-H.; Huang, C.-W.; Wu, J. Hydrogen production from semiconductor-based photocatalysis via water splitting. Catalysts 2012, 2, 490–516. [Google Scholar] [CrossRef]
- Huang, C.-W.; Liao, C.-H.; Wu, J.C. Photocatalytic separate evolution of hydrogen and oxygen over highly ordered nanorods and bulk TiO2 thin films. J. Clean Energy Technol. 2013, 1, 1–5. [Google Scholar] [CrossRef]
- Al-Mazroai, L.S.; Bowker, M.; Davies, P.; Dickinson, A.; Greaves, J.; James, D.; Millard, L. The photocatalytic reforming of methanol. Catal. Today 2007, 122, 46–50. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.; Leung, D.Y.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energ Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Nosaka, Y.; Nosaka, A.Y. Generation and detection of reactive oxygen species in photocatalysis. Chem. Rev. 2017, 117, 11302–11336. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Kim, S.; Bard, A.J. Novel Carbon-Doped TiO2 Nanotube Arrays with High Aspect Ratios for Efficient Solar Water Splitting. Nano Lett. 2006, 6, 24–28. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.-C.; Hsiao, K.-C.; Chang, Y.-H.; Chan, S.-H. Photocatalytic hydrogen evolution of palladium nanoparticles decorated black TiO2 calcined in argon atmosphere. Appl. Surf. Sci. 2018, 430, 407–414. [Google Scholar] [CrossRef]
- Lei, P.; Wang, F.; Zhang, S.; Ding, Y.; Zhao, J.; Yang, M. Conjugation-grafted-TiO2 nanohybrid for high photocatalytic efficiency under visible light. ACS Appl. Mater. Interface 2014, 6, 2370–2376. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Wei, T.; Zhang, Y.; Song, X.; Huan, Y.; Liu, H.; Zhao, M.; Yu, J.; Chen, X. A Photoresponsive Rutile TiO2 Heterojunction with Enhanced Electron–Hole Separation for High-Performance Hydrogen Evolution. Adv. Mater. 2019, 31, 1806596. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Hong, B.-Y.; Tseng, C.-M. Sol–gel preparation and photocatalysis of titanium dioxide. Catal. Today 2004, 96, 119–126. [Google Scholar] [CrossRef]
- Kudo, A.; Miseki, Y. Heterogeneous photocatalyst materials for water splitting. Chem. Soc. Rev. 2009, 38, 253–278. [Google Scholar] [CrossRef]
- Anpo, M.; Takeuchi, M. The design and development of highly reactive titanium oxide photocatalysts operating under visible light irradiation. J. Catal. 2003, 216, 505–516. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Dong, X.; Zheng, S.; Dionysiou, D.D. Acetic acid functionalized TiO2/kaolinite composite photocatalysts with enhanced photocatalytic performance through regulating interfacial charge transfer. J. Catal. 2018, 367, 126–138. [Google Scholar] [CrossRef]
- Gao, B.; Yap, P.S.; Lim, T.M.; Lim, T.-T. Adsorption-photocatalytic degradation of Acid Red 88 by supported TiO2: Effect of activated carbon support and aqueous anions. Chem. Eng. J. 2011, 171, 1098–1107. [Google Scholar] [CrossRef]
- Lu, J.; Li, L.; Wang, Z.; Wen, B.; Cao, J. Synthesis of visible-light-active TiO2-based photo-catalysts by a modified sol–gel method. Mater. Lett. 2013, 94, 147–149. [Google Scholar] [CrossRef]
- Alkaim, A.F.; Kandiel, T.A.; Hussein, F.H.; Dillert, R.; Bahnemann, D.W. Solvent-free hydrothermal synthesis of anatase TiO2 nanoparticles with enhanced photocatalytic hydrogen production activity. Appl. Catal. A 2013, 466, 32–37. [Google Scholar] [CrossRef]
- Liu, S.-H.; Syu, H.-R. One-step fabrication of N-doped mesoporous TiO2 nanoparticles by self-assembly for photocatalytic water splitting under visible light. Appl. Energ 2012, 100, 148–154. [Google Scholar] [CrossRef]
- Liu, N.; Albu, S.P.; Lee, K.; So, S.; Schmuki, P. Water annealing and other low temperature treatments of anodic TiO2 nanotubes: A comparison of properties and efficiencies in dye sensitized solar cells and for water splitting. Electrochim. Acta 2012, 82, 98–102. [Google Scholar] [CrossRef]
- Llano, B.; Hidalgo, M.; Rios, L.; Navio, J. Effect of the type of acid used in the synthesis of titania–silica mixed oxides on their photocatalytic properties. Appl. Catal. B 2014, 150, 389–395. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Selim, A.Q.; Mohamed, E.A.; Seliem, M.K.; Zayed, A.M. Synthesis of sole cancrinite phase from raw muscovite: Characterization and optimization. J. Alloy Compd. 2018, 762, 653–667. [Google Scholar] [CrossRef]
- Shahravan, A.; Desai, T.; Matsoukas, T. Controlled manipulation of wetting characteristics of nanoparticles with dry-based plasma polymerization method. Appl. Phys. Lett. 2012, 101, 251603. [Google Scholar] [CrossRef]
- Socrates, G. Infrared and Raman Characteristic Group Frequencies: Tables and Charts; John Wiley & Sons: Baffins Lane, UK, 2004. [Google Scholar]
- Fujimoto, M.; Ohno, T.; Suzuki, H.; Koyama, H.; Tanaka, J. Nanostructure of TiO2 Nano-Coated SiO2 Particles. J. Am. Ceram. Soc. 2005, 88, 3264–3266. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Lei, D.; He, Y.-B.; Li, B.; Kang, F. Acetic acid-induced preparation of anatase TiO2 mesocrystals at low temperature for enhanced Li-ion storage. J. Mater. Chem. A 2017, 5, 12236–12242. [Google Scholar] [CrossRef]
- Ren, Y.; Zhao, L.; Zou, Y.; Song, L.; Dong, N.; Wang, J. Effects of Different TiO2 Particle Sizes on the Microstructure and Optical Limiting Properties of TiO2/Reduced Graphene Oxide Nanocomposites. Nanomaterial 2019, 9, 730. [Google Scholar] [CrossRef] [PubMed]
- Sahu, D.R.; Hong, L.; Wang, S.-C.; Huang, J.-L. Synthesis, analysis and characterization of ordered mesoporous TiO2/SBA-15 matrix: Effect of calcination temperature. Microporous Mesoporous Mater. 2009, 117, 640–649. [Google Scholar] [CrossRef]
- Shiyanovskaya, I.; Hepel, M. Decrease of Recombination Losses in Bicomponent WO3/TiO2 Films Photosensitized with Cresyl Violet and Thionine. J. Electrochem. Soc. 1998, 145, 3981–3985. [Google Scholar] [CrossRef]
- Nakade, S.; Saito, Y.; Kubo, W.; Kitamura, T.; Wada, Y.; Yanagida, S. Influence of TiO2 nanoparticle size on electron diffusion and recombination in dye-sensitized TiO2 solar cells. J. Phys. Chem. B 2003, 107, 8607–8611. [Google Scholar] [CrossRef]
- Li, D.; Yu, J.C.-C.; Nguyen, V.-H.; Wu, J.C.S.; Wang, X. A dual-function photocatalytic system for simultaneous separating hydrogen from water splitting and photocatalytic degradation of phenol in a twin-reactor. Appl. Catal. B 2018, 239, 268–279. [Google Scholar] [CrossRef]
- Fontelles-Carceller, O.; Muñoz-Batista, M.J.; Conesa, J.C.; Kubacka, A.; Fernández-García, M. H2 photo-production from methanol, ethanol and 2-propanol: Pt-(Nb)TiO2 performance under UV and visible light. Mol. Catal. 2018, 446, 88–97. [Google Scholar] [CrossRef]
- Yang, X.; Salzmann, C.; Shi, H.; Wang, H.; Green, M.L.H.; Xiao, T. The Role of Photoinduced Defects in TiO2 and Its Effects on Hydrogen Evolution from Aqueous Methanol solution. J. Phys. Chem. A 2008, 112, 10784–10789. [Google Scholar] [CrossRef] [PubMed]
- Chiarello, G.L.; Aguirre, M.H.; Selli, E. Hydrogen production by photocatalytic steam reforming of methanol on noble metal-modified TiO2. J. Catal. 2010, 273, 182–190. [Google Scholar] [CrossRef]
- Majrik, K.; Turcsányi, Á.; Pászti, Z.; Szabó, T.; Domján, A.; Mihály, J.; Tompos, A.; Dékány, I.; Tálas, E. Graphite Oxide-TiO2 Nanocomposite Type Photocatalyst for Methanol Photocatalytic Reforming Reaction. Top. Catal. 2018, 61, 1323–1334. [Google Scholar] [CrossRef]
- Mills, A.; Bingham, M.; O’Rourke, C.; Bowker, M. Modelled kinetics of the rate of hydrogen evolution as a function of metal catalyst loading in the photocatalysed reforming of methanol by Pt (or Pd)/TiO2. J. Photochem. Photobiol. A 2019, 373, 122–130. [Google Scholar] [CrossRef]
- Jones, W.; Martin, D.J.; Caravaca, A.; Beale, A.M.; Bowker, M.; Maschmeyer, T.; Hartley, G.; Masters, A. A comparison of photocatalytic reforming reactions of methanol and triethanolamine with Pd supported on titania and graphitic carbon nitride. Appl. Catal. B 2019, 240, 373–379. [Google Scholar] [CrossRef]
- Han, B.; Hu, Y.H. Highly Efficient Temperature-Induced Visible Light Photocatalytic Hydrogen Production from Water. J. Phys. Chem. C 2015, 119, 18927–18934. [Google Scholar] [CrossRef]
- Gu, Q.; Long, J.; Zhuang, H.; Zhang, C.; Zhou, Y.; Wang, X. Ternary Pt/SnOx/TiO2 photocatalysts for hydrogen production: Consequence of Pt sites for synergy of dual co-catalysts. Phys. Chem. Chem. Phys. 2014, 16, 12521–12534. [Google Scholar] [CrossRef] [PubMed]
- Tálas, E.; Pászti, Z.; Korecz, L.; Domján, A.; Németh, P.; Szíjjártó, G.P.; Mihály, J.; Tompos, A. PtOx-SnOx-TiO2 catalyst system for methanol photocatalytic reforming: Influence of cocatalysts on the hydrogen production. Catal. Today 2018, 306, 71–80. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Li, Y.-L.; Jiu, B.-B.; Gong, F.-L.; Chen, J.-L.; Fang, S.-M.; Zhang, H.-L. Highly enhanced photocatalytic H2 evolution of Cu2O microcube by coupling with TiO2 nanoparticles. Nanotechnology 2019, 30, 145401. [Google Scholar] [CrossRef] [PubMed]
- Xu, F.; Zhang, L.; Cheng, B.; Yu, J. Direct Z-Scheme TiO2/NiS Core–Shell Hybrid Nanofibers with Enhanced Photocatalytic H2-Production Activity. ACS Sustain. Chem. Eng. 2018, 6, 12291–12298. [Google Scholar] [CrossRef]
- Xin, Y.; Lu, Y.; Han, C.; Ge, L.; Qiu, P.; Li, Y.; Fang, S. Novel NiS cocatalyst decorating ultrathin 2D TiO2 nanosheets with enhanced photocatalytic hydrogen evolution activity. Mater. Res. Bull. 2017, 87, 123–129. [Google Scholar] [CrossRef]








| Addition of Acetic Acid (mL) | TiO2 Photocatalysts | |
|---|---|---|
| w/o Support | Loaded on ST-3000 | |
| 0 | T | TS |
| 1 | 1A-T | 1A-TS |
| 5 | 5A-T | 5A-TS |
| 10 | 10A-T | 10A-TS |
| Atomic (%) | Ti (%) | O (%) | Al (%) | Si (%) | K (%) | Ti/Si | SBET (m2/g) |
|---|---|---|---|---|---|---|---|
| T | 31.32 | 68.68 | <L.D. | <L.D. | <L.D. | - | 31.9 |
| 1A-T | 32 | 68 | <L.D. | <L.D. | <L.D. | - | - |
| 5A-T | 30.42 | 69.58 | <L.D. | <L.D. | <L.D. | - | - |
| 10A-T | 29.69 | 70.31 | <L.D. | <L.D. | <L.D. | - | 58.2 |
| TS | 5.71 | 70.42 | 9.16 | 13.23 | 1.48 | 0.43 | 39.9 |
| 1A-TS | 13.2 | 59.68 | 10.52 | 13.73 | 2.86 | 0.96 | - |
| 5A-TS | 9.53 | 63.36 | 11.04 | 13.54 | 2.54 | 0.7 | - |
| 10A-TS | 9.86 | 68.19 | 9.14 | 10.8 | 2.01 | 0.91 | 38.8 |
| Sericite | <L.D. | 71.65 | 11.15 | 15.54 | 1.66 | 0 | 7.8 |
| P25 | N.A. | 52 | |||||
| No. | Photocatalysts | Light Source | Reactant Medium | H2 Evolution/ μmol/g·h | Ref. (Year) |
|---|---|---|---|---|---|
| 1 | TiO2 (P25) | UV lamp (200 W), λ = 320–500 nm | CH3OH/H2O (l, 1:9) | 202 | This study |
| 2 | TiO2 (10A-T) | 1150 | |||
| 3 | TiO2/sericite (10A-TS) | 1424 | |||
| 4 | Pt/TiO2 | Hg-Xe lamp (500 W) integrated dichroic filters (LOT Quantum Design, λ = 280–400 nm) | CH3OH/H2O (l, 3:7) | 1602 | [38] (2018) |
| 5 | Pt/TiO2 | Hg-Xe lamp (500 W) integrated dichroic filters (LOT Quantum Design, λ = 420–680 nm) | 386 | ||
| 6 | TiO2 | Xe lamp (PLS-SXE300, 300 W) | CH3OH/H2O (l, 1:9) | 85 | [39] (2008) |
| 7 | TiO2 | Iron halogenide Hg arc lamp (Jelosil, 250 W): λ = 350–450 nm; 37 mW/cm2 | CH3OH/H2O/N2 (g, 2:3:95, 40 mL/min) | 360 | [40] (2010) |
| 8 | FP-TiO2 | 720 | |||
| 9 | 1%Ag/TiO2 | 1170 | |||
| 10 | 1%Au/TiO2 | 13,300 | |||
| 11 | TiO2 | Osram HQL deluxe lamps (125 W) | CH3OH (aq, 6v.%) | 120 | [41] (2018) |
| 12 | 0.5%Pt/TiO2 | 9290 | |||
| 13 | 1%Ag/TiO2 | 300 | |||
| 14 | 1%GO/TiO2 | 391 | |||
| 15 | 2%GO/TiO2 | 502 | |||
| 16 | 10%GO/TiO2 | 439 | |||
| 17 | 0.001%Pt/TiO2 | UV LED (2.8 W): λ = 365 nm; 20 mW/cm2 | CH3OH/H2O (l, 1:1) | 270 | [42] (2019) |
| 18 | 0.01%Pt/TiO2 | 1970 | |||
| 19 | 0.05%Pt/TiO2 | 3330 | |||
| 20 | 0.2%Pt/TiO2 | 6475 | |||
| 21 | 1%Pt/TiO2 | 5725 | |||
| 22 | 10%Pt/TiO2 | 1145 | |||
| 23 | 0.5%Pd/TiO2 | Xe arc lamp (LOT-Oriel, 150W) | CH3OH (aq, 0.125 M) | 580 | [43] (2019) |
| 24 | 1%Pt/TiO2 | Xe lamp (Newport, an AM 1.5G filter, 150 W): 100 mW/cm2 | CH3OH/H2O (l, 3:7) | 200 | [44] (2015) |
| 25 | Pt3.0/TiO2 | High-pressure Hg lamp (125 W): λ = 365 nm, 1.5 W/cm2 | CH3OH/H2O (l, 1:33) | 1560 | [45] (2014) |
| 26 | PtOx-SnOx/TiO2 | Osram HQL deluxe lamps (125 W) | CH3OH (aq, 6v.%) | 2000 | [46] (2018) |
| 27 | Cu2O/TiO2 | Xe lamp (300 W) | CH3OH/H2O (l, 1:4) | 500.4 | [47] (2019) |
| 28 | NiS/TiO2 nanofibers | Xe arc lamp (XD350, 350 W) | CH3OH/H2O (l, 1:4) | 655 | [48] (2018) |
| 29 | 5%NiS/TiO2 nanosheets | UV Xe lamp (300 W): λ > 300 nm | CH3OH/H2O (l, 1:3) | 313.6 | [49] (2016) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-W.; Wu, M.-C.; Nguyen, V.-H.; Nguyen, B.-S. Preparation and Characterization of Nanocrystalline TiO2 on Microsericite for High-Efficiency Photo-Energy Conversion of Methanol to Hydrogen. Crystals 2019, 9, 380. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080380
Huang C-W, Wu M-C, Nguyen V-H, Nguyen B-S. Preparation and Characterization of Nanocrystalline TiO2 on Microsericite for High-Efficiency Photo-Energy Conversion of Methanol to Hydrogen. Crystals. 2019; 9(8):380. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080380
Chicago/Turabian StyleHuang, Chao-Wei, Min-Chien Wu, Van-Huy Nguyen, and Ba-Son Nguyen. 2019. "Preparation and Characterization of Nanocrystalline TiO2 on Microsericite for High-Efficiency Photo-Energy Conversion of Methanol to Hydrogen" Crystals 9, no. 8: 380. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080380