Argentophilic Interactions in Two AgI Complexes of 3-(2-(Pyridin-4-yl)ethyl)pentane-2,4-dione, a Promising Ditopic Ligand
Abstract
:1. Introduction
2. Experimental Section
2.1. Methods and Materials
2.2. Synthesis of 3-(2-(Pyridin-4-yl)ethyl)pentane-2,4-dione (HacacPyen)
2.3. Synthesis and Crystallization of [Ag(HacacPyen)2]PF6, 1
2.4. Synthesis and Crystallization of [Ag(HacacPyen)2]BF4·C4H8O2, 2
2.5. Structure Determinations
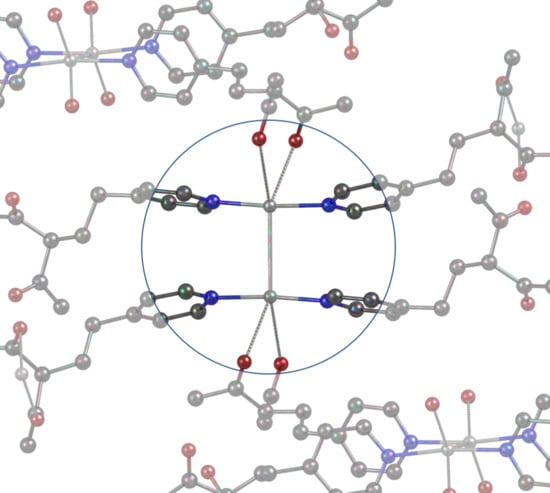
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| HacacCN | 3-cyanopentane-2,4-dione |
| HacacPy | 3-(pyridin-4-yl)pentane-2,4-dione |
| HacacPyen | 3-(2-(pyridin-4-yl)ethyl)pentane-2,4-dione |
References
- Kurmoo, M. Magnetic metal-organic frameworks. Chem. Soc. Rev. 2009, 38, 1353–1379. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.N.; Zhou, M.; Li, S.; Li, Z.; Li, J.; Wu, B.; Li, G.; Li, F.; Guan, X. Magnetic metal-organic frameworks: γ-Fe2O3@MOFs via confined in situ pyrolysis method for drug delivery. Small 2014, 10, 2927–2936. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y.; Yue, Y.; Qian, G.; Chen, B. Luminescent functional metal-organic frameworks. Chem. Rev. 2012, 112, 1126–1162. [Google Scholar] [CrossRef] [PubMed]
- Li, J.R.; Sculley, J.; Zhou, H.C. Metal-organic frameworks for separations. Chem. Rev. 2012, 112, 869–932. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Farha, O.K.; Roberts, J.; Scheidt, K.A.; Nguyen, S.T.; Hupp, J.T. Metal-organic framework materials as catalysts. Chem. Soc. Rev. 2009, 38, 1450–1459. [Google Scholar] [CrossRef]
- Ma, L.; Falkowski, J.M.; Abney, C.; Lin, W. A series of isoreticular chiral metal-organic frameworks as a tunable platform for asymmetric catalysis. Nat. Chem. 2010, 2, 838–846. [Google Scholar] [CrossRef]
- Bansal, D.; Pandey, S.; Hundal, G.; Gupta, R. Heterometallic coordination polymers: syntheses, structures and heterogeneous catalytic applications. New J. Chem. 2015, 39, 9772–9781. [Google Scholar] [CrossRef]
- Konkol, M.; Kondracka, M.; Kowalik, P.; Próchniak, W.; Michalska, K.; Schwedt, A.; Merkens, C.; Englert, U. Decomposition of the mixed-metal coordination polymer—A preparation route of the active Ag/Yb2O3 catalyst for the deN2O process. Appl. Catal. B—Environ. 2016, 190, 85–92. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases. J. Am. Chem. Soc. 1963, 85, 3533–3539. [Google Scholar] [CrossRef]
- Pearson, R.G. Hard and Soft Acids and Bases, HSAB, Part 1: Fundamental principles. J. Chem. Educ. 1968, 45, 581–587. [Google Scholar] [CrossRef]
- Mackay, L.G.; Anderson, H.L.; Sanders, J.K.M. A platinum-linked porphyrin trimer and a complementary aluminium tris[3-(4-pyridyl)acetylacetonate] guest. J. Chem. Soc. Perkin Trans. 1995, 18, 2269–2273. [Google Scholar] [CrossRef]
- Vreshch, V.D.; Chernega, A.N.; Howard, J.A.K.; Sieler, J.; Domasevitch, K.V. Two-step construction of molecular and polymeric mixed-metal Cu(Co)/Be complexes employing functionality of a pyridyl substituted acetylacetonate. Dalton Trans. 2003, 1707–1711. [Google Scholar] [CrossRef]
- Vreshch, V.D.; Lysenko, A.B.; Chernega, A.N.; Howard, J.A.K.; Krautscheid, H.; Sieler, J.; Domasevitch, K.V. Extended coordination frameworks incorporating heterobimetallic squares. Dalton Trans. 2004, 2899–2903. [Google Scholar] [CrossRef] [PubMed]
- Vreshch, V.D.; Lysenko, A.B.; Chernega, A.N.; Sieler, J.; Domasevitch, K.V. Heterobimetallic Cd(Zn)/Be coordination polymers involving pyridyl functionalized beryllium diketonates. Polyhedron 2005, 24, 917–926. [Google Scholar] [CrossRef]
- Chen, B.; Fronczek, F.R.; Maverick, A.W. Solvent-dependent 44 square grid and 64.82 NbO frameworks formed by Cu(Pyac)2 (bis[3-(4-pyridyl)pentane-2,4-dionato]copper(ii)). Chem. Commun. 2003, 2166–2167. [Google Scholar] [CrossRef]
- Chen, B.; Fronczek, F.R.; Maverick, A.W. Porous Cu-Cd mixed-metal-organic frameworks constructed from Cu(Pyac)2 Bis{3-(4-pyridyl)pentane-2,4-dionatocopper(II)}. Inorg. Chem. 2004, 43, 8209–8211. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, B.; Fronczek, F.R.; Maverick, A.W. A nanoporous Ag-Fe mixed-metal-organic framework exhibiting single-crystal-to-single-crystal transformations upon guest exchange. Inorg. Chem. 2008, 47, 4433–4435. [Google Scholar] [CrossRef]
- Li, D.J.; Mo, L.Q.; Wang, Q.M. Heterometallic coordination polymers generated from tripodal metalloligands. Inorg. Chem. Commun. 2011, 14, 1128–1131. [Google Scholar] [CrossRef]
- Merkens, C.; Truong, K.N.; Englert, U. 3-(4-pyridyl)-acetylacetone—A fully featured substituted pyridine and a flexible linker for complex materials. Acta Crystallogr. 2014, B70, 705–713. [Google Scholar] [CrossRef]
- Truong, K.N.; Merkens, C.; Meven, M.; Faßbänder, B.; Dronskowski, R.; Englert, U. Phase transition and proton ordering at 50 K in 3-(pyridin-4-yl)pentane-2,4-dione. Acta Crystallogr. 2017, B73, 1172–1178. [Google Scholar] [CrossRef]
- Fackler, J.P. The C≡N Stretching Frequency in Metal Complexes of 3-Cyanopentane-2,4-dione. J. Chem. Soc. 1962, 1957–1960. [Google Scholar] [CrossRef]
- Silvernail, C.M.; Yap, G.; Sommer, R.D.; Rheingold, A.L.; Day, V.W.; Belot, J.A. An effective synthesis of alkyl β-cyano-α,γ-diketones using chlorosulfonylisocyanate and a representative Cu(II) complex. Polyhedron 2001, 20, 3113–3117. [Google Scholar] [CrossRef]
- Balahura, R.J.; Ferguson, G.; Johnston, A.; Ruhl, B.L. Reactions and X-ray crystal structure of (3-cyano-2,4-pentanedionato-N)pentamminecobalt(III) perchlorate chloride dihydrate. Polyhedron 1986, 5, 2075–2080. [Google Scholar] [CrossRef]
- Tsiamis, C.; Tzavellas, L.C.; Stergiou, A.; Anesti, V. Variable Coordination and Conformation of the 3-Cyano-2,4-pentanedionato Anion in a Mixed-Ligand Binuclear Copper(II) Chelate. Inorg. Chem. 1996, 35, 4984–4988. [Google Scholar] [CrossRef]
- Burrows, A.D.; Cassar, K.; Mahon, M.F.; Warren, J.E. The stepwise formation of mixed-metal coordination networks using complexes of 3-cyanoacetylacetonate. Dalton Trans. 2007, 2499–2509. [Google Scholar] [CrossRef]
- Kondracka, M.; Englert, U. Bimetallic coordination polymers via combination of substitution-inert building blocks and labile connectors. Inorg. Chem. 2008, 47, 10246–10257. [Google Scholar] [CrossRef]
- Pogozhev, D.; Baudron, S.A.; Hosseini, M.W. Assembly of heteroleptic copper complexes with silver salts: From discrete trinuclear complexes to infinite networks. Inorg. Chem. 2010, 49, 331–338. [Google Scholar] [CrossRef]
- Kilduff, B.; Pogozhev, D.; Baudron, S.A.; Hosseini, M.W. Heterometallic architectures based on the combination of heteroleptic copper and cobalt complexes with silver salts. Inorg. Chem. 2010, 49, 11231–11239. [Google Scholar] [CrossRef]
- Merkens, C.; Becker, N.; Lamberts, K.; Englert, U. Bimetallic coordination networks based on Al(acacCN)3: A building block between inertness and lability. Dalton Trans. 2012, 41, 8594–8599. [Google Scholar] [CrossRef]
- Merkens, C.; Englert, U. Ordered bimetallic coordination networks featuring rare earth and silver cations. Dalton Trans. 2012, 41, 4664–4673. [Google Scholar] [CrossRef]
- Wang, A.; Merkens, C.; Englert, U. Interplay of ligand chirality and metal configuration in mononuclear complexes and in a coordination polymer of Cr(iii). CrystEngComm 2015, 17, 4293–4300. [Google Scholar] [CrossRef] [Green Version]
- Guo, Q.; Merkens, C.; Si, R.; Englert, U. Crosslinking of the Pd(acacCN)2 building unit with Ag(i) salts: Dynamic 1D polymers and an extended 3D network. CrystEngComm 2015, 17, 4383–4393. [Google Scholar] [CrossRef]
- Guo, Q.; Englert, U. Neutral mixed-metal coordination polymers based on a ditopic acetylacetonate, Mg(ii) and Ag(i): Syntheses, characterization and solvent-dependent topologies. Dalton Trans. 2017, 46, 8514–8523. [Google Scholar] [CrossRef]
- Kremer, M.; Englert, U. N Donor substituted acetylacetones—Versatile ditopic ligands. Z. Krist.—Cryst. Mater. 2018, 233, 437–452. [Google Scholar] [CrossRef]
- Truong, K.N.; Meven, M.; Englert, U. Proton disorder in a short intramolecular hydrogen bond investigated by single-crystal neutron diffraction at 2.5 and 170 K. Acta Crystallogr. 2018, C74, 1635–1640. [Google Scholar] [CrossRef]
- Bruker. SAINT+: Program for Reduction of Data Collected on Bruker CCD Area Detector Diffractometer; Bruker: Madison, WI, USA, 2009. [Google Scholar]
- Bruker. SADABS; Bruker: Madison, WI, USA, 2008. [Google Scholar]
- Sheldrick, G.M. SHELXT—Integrated space-group and crystal-structure determination. Acta Crystallogr. 2015, A71, 3–8. [Google Scholar] [CrossRef]
- Spek, A.L. PLATON SQUEEZE: A tool for the calculation of the disordered solvent contribution to the calculated structure factors. Acta Crystallogr. 2015, C71, 9–18. [Google Scholar] [CrossRef]
- CCDC 1936466 Contains the Supplementary Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 8 August 2019).
- CCDC 1944686 Contains the Supplementary Crystallographic Data for This Paper. Available online: http://www.ccdc.cam.ac.uk/conts/retrieving.html (accessed on 8 August 2019).
- Spek, A.L. Structure validation in chemical crystallography. Acta Crystallogr. 2009, D65, 148–155. [Google Scholar] [CrossRef]
- Kalf, I.; Braun, M.; Wang, Y.; Englert, U. Homo- and heterochiral coordination polymers of silver with diaminocyclohexane as bridging ligand: The effect of chirality on argentophilic interactions. CrystEngComm 2006, 8, 916. [Google Scholar] [CrossRef]
- Bondi, A. van der Waals Volumes and Radii. J. Phys. Chem. 1964, 68, 441–451. [Google Scholar] [CrossRef]
- Batsanov, S.S. Van der Waals radii of elements from the data of structural inorganic chemistry. Russ. Chem. Bull. 1995, 44, 2245–2250. [Google Scholar] [CrossRef]
- Omary, M.A.; Webb, T.R.; Assefa, Z.; Shankle, G.E.; Patterson, H.H. Crystal Structure, Electronic Structure, and Temperature-Dependent Raman Spectra of Tl[Ag(CN)2]: Evidence for Ligand-Unsupported Argentophilic Interactions. Inorg. Chem. 1998, 37, 1380–1386. [Google Scholar] [CrossRef]
- Chu, Q.; Swenson, D.C.; MacGillivray, L.R. A single-crystal-to-single-crystal transformation mediated by argentophilic forces converts a finite metal complex into an infinite coordination network. Angew. Chem. Int. Ed. 2005, 44, 3569–3572. [Google Scholar] [CrossRef]
- Dobrzańska, L.; Raubenheimer, H.G.; Barbour, L.J. Borromean sheets assembled by self-supporting argentophilic interactions. Chem. Commun. 2005, 5050–5052. [Google Scholar] [CrossRef]
- Park, B.I.; Lee, J.W.; Lee, Y.A.; Hong, J.; Jung, O.S. Structural Properties of AgX Bearing Bis(3-pyridyl)dimethylsilane (X− = CF3SO3−, PF6−, and NO3−). Bull. Chem. Soc. Jpn. 2005, 78, 1624–1628. [Google Scholar] [CrossRef]
- Liu, X.; Guo, G.C.; Fu, M.L.; Liu, X.H.; Wang, M.S.; Huang, J.S. Three novel silver complexes with ligand-unsupported argentophilic interactions and their luminescent properties. Inorg. Chem. 2006, 45, 3679–3685. [Google Scholar] [CrossRef]
- Wang, Y.; Şerb, M.; Englert, U. Silver coordination polymers with remarkably high packing coefficients. Struct. Chem. 2010, 21, 203–211. [Google Scholar] [CrossRef]
- Wang, Y.; Englert, U. Homo- and heterochiral coordination polymers of silver with diaminocyclohexane as bridging ligand: Trends in alkylbenzoates. Inorg. Chim. Acta 2010, 363, 2539–2545. [Google Scholar] [CrossRef]
- Kalf, I.; Mathieu, P.; Englert, U. From crystal to crystal: A new polymorph of (4-carboxylatopyridine)silver(i) by topotactic dehydration of its monohydrate. New J. Chem. 2010, 34, 2491. [Google Scholar] [CrossRef]
- Guo, Q.; Englert, U. An Acetylacetonate or a Pyrazole? Both! 3-(3,5-Dimethyl-pyrazol-4-yl)pentane-2,4-dione as a Ditopic Ligand. Cryst. Growth Des. 2016, 16, 5127–5135. [Google Scholar] [CrossRef]
- Schmidbaur, H.; Schier, A. Argentophilic interactions. Angew. Chem. Int. Ed. 2015, 54, 746–784. [Google Scholar] [CrossRef]
- Pyykkö, P. Strong Closed-Shell Interactions in Inorganic Chemistry. Chem. Rev. 1997, 97, 597–636. [Google Scholar] [CrossRef]
- Jung, O.S.; Kim, Y.J.; Lee, Y.A.; Park, K.M.; Lee, S.S. Subtle role of polyatomic anions in molecular construction: structures and properties of AgX bearing 2,4′-thiobis(pyridine) (X− = NO3−, BF4−, ClO4−, PF6−, CF3CO2−, and CF3SO3−). Inorg. Chem. 2003, 42, 844–850. [Google Scholar] [CrossRef]
- Lee, J.W.; Kim, E.A.; Kim, Y.J.; Lee, Y.A.; Pak, Y.; Jung, O.S. Relationship between the ratio of ligand to metal and the coordinating ability of anions. Synthesis and structural properties of AgX-bearing bis(4-pyridyl)dimethylsilane (X− = NO2−, NO3−, CF3SO3−, and PF6−). Inorg. Chem. 2005, 44, 3151–3155. [Google Scholar] [CrossRef]
- Chen, X.D.; Mak, T.C.W. Order of the coordinating ability of polyatomic monoanions established from their interaction with a disilver(I) metallacyclophane skeleton. Chem. Commun. 2005, 3529–3531. [Google Scholar] [CrossRef]






| O1–C2 / Å | 1.221(6) | O3–C14 / Å | 1.201(5) |
| O2–C4 / Å | 1.233(6) | O4–C16 / Å | 1.221(6) |
| C2–C3 / Å | 1.508(6) | C14–C15 / Å | 1.528(6) |
| C3–C4 / Å | 1.525(7) | C15–C16 / Å | 1.512(6) |
| ∠(C2–C3–C4) / ° | 109.1(4) | ∠(C14–C15–C16) / ° | 108.7(4) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
van Terwingen, S.; Englert, U. Argentophilic Interactions in Two AgI Complexes of 3-(2-(Pyridin-4-yl)ethyl)pentane-2,4-dione, a Promising Ditopic Ligand. Crystals 2019, 9, 414. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080414
van Terwingen S, Englert U. Argentophilic Interactions in Two AgI Complexes of 3-(2-(Pyridin-4-yl)ethyl)pentane-2,4-dione, a Promising Ditopic Ligand. Crystals. 2019; 9(8):414. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080414
Chicago/Turabian Stylevan Terwingen, Steven, and Ulli Englert. 2019. "Argentophilic Interactions in Two AgI Complexes of 3-(2-(Pyridin-4-yl)ethyl)pentane-2,4-dione, a Promising Ditopic Ligand" Crystals 9, no. 8: 414. https://0-doi-org.brum.beds.ac.uk/10.3390/cryst9080414