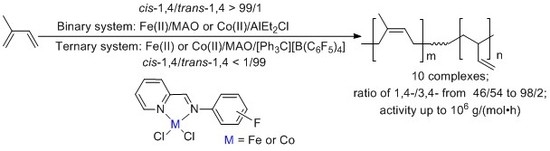
Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization
Abstract
:1. Introduction
2. Materials and Methods
2.1. General Information
2.2. Experimental Methods
2.2.1. Synthesis of 1-(Pyridin-2-yl)-N-(2,4,6-trifluorophenyl)methanimine (L3)
2.2.2. Synthesis of Iminopyridine Fe(II) Complexes 1a, 2a, 3a
Synthesis of 1a
Synthesis of 2a
Synthesis of 3a
2.2.3. Synthesis of Iminopyridine Co(II) Complexes 1b, 2b, 3b
Synthesis of 1b
Synthesis of 2b
Synthesis of 3b
2.2.4. General Procedure for Isoprene Polymerization
3. Results and Discussion
3.1. The Synthesis and Characterization of Iminopyridine Ligands and Corresponding Fe(II) and Co(II) Complexes
3.2. Isoprene Polymerization Studies
3.2.1. Polymerization of Isoprene with Fe(II) Catalysts
3.2.2. Polymerization of Isoprene with Co(II) Catalysts
3.2.3. The Ternary System of Iminopyridine Fe(II) and Co(II)-Catalyzed Isoprene Polymerization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Ouardad, S.; Bakleh, M.-E.; Kostjuk, S.V.; Ganachaud, F.; Puskas, J.E.; Deffieux, A.; Peruch, F. Bio-inspired cationic polymerization of isoprene and analogues: State-of-the-art. Polym. Int. 2012, 61, 149–156. [Google Scholar] [CrossRef]
- Horne, S.E.; Kiehl, J.P.; Shipman, J.J.; Folt, V.L.; Gibbs, C.F.; Willson, E.A.; Newton, E.B.; Reinhart, M.A.; Willson, E.A. Ameripol SN—A cis-1,4-polyisoprene. Ind. Eng. Chem. 1956, 48, 784–791. [Google Scholar] [CrossRef]
- Ricci, G.; Sommazzi, A.; Masi, F.; Ricci, M.; Boglia, A.; Leone, G. Well-defined transition metal complexes with phosphorus and nitrogen ligands for 1,3-dienes polymerization. Coord. Chem. Rev. 2010, 254, 661–676. [Google Scholar] [CrossRef]
- Wang, B.L.; Cui, D.M.; Lv, K. Highly 3,4-selective living polymerization of isoprene with rare earth metal fluorenyl N-heterocyclic carbene precursors. Macromolecules 2008, 41, 1983–1988. [Google Scholar] [CrossRef]
- Kamienski, C.W. Lithium catalysis in industrial polymerization. Ind. Eng. Chem. 1965, 57, 38–55. [Google Scholar] [CrossRef]
- Lopez-Sanchez, J.A.; Lamberti, M.; Pappalardo, D.; Pellecchia, C. Polymerization of conjugated dienes promoted by bis(phenoxyimino)titanium catalysts. Macromolecules 2003, 36, 9260–9263. [Google Scholar] [CrossRef]
- Osakada, K.; Takeuchi, D. Coordination polymerization of dienes, allenes, and methylenecycloalkanes. Polym. Synth. 2004, 171, 137–194. [Google Scholar]
- Zhang, L.X.; Suzuki, T.; Luo, Y.; Nishiura, M.; Hou, Z.M. Cationic alkyl rare-earth metal complexes bearing an ancillary bis(phosphinophenyl)amido ligand: A catalytic system for living cis-1,4-polymerization and copolymerization of isoprene and butadiene. Angew. Chem. Int. Ed. 2007, 46, 1909–1913. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Cui, D.M. Highly cis-1,4 selective polymerization of dienes with homogeneous Ziegler-Natta catalysts based on NCN-pincer rare earth metal dichioride precursors. J. Am. Chem. Soc. 2008, 130, 4984–4991. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.X.; Nishiura, M.; Yuki, M.; Luo, Y.; Hou, Z.M. Isoprene polymerization with yttrium amidinate catalysts: Switching the regio- and stereoselectivity by addition of AlMe3. Angew. Chem. Int. Ed. 2008, 47, 2642–2645. [Google Scholar] [CrossRef] [PubMed]
- Lv, K.; Cui, D.M. CCC-pincer bis(carbene) lanthanide dibromides. Catalysis on highlycis-1,4-selective polymerization of isoprene and active species. Organometallics 2010, 29, 2987–2993. [Google Scholar] [CrossRef]
- Li, D.F.; Li, S.H.; Cui, D.M.; Zhang, X.Q. β-diketiminato rare-earth metal complexes. structures, catalysis, and active species for highly cis-1,4-selective polymerization of isoprene. Organometallics 2010, 29, 2186–2193. [Google Scholar] [CrossRef]
- Nishiura, M.; Hou, Z.M. Novel polymerization catalysts and hydride clusters from rare-earth metal dialkyls. Nat. Chem. 2010, 2, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Britovsek, G.J.P.; Gibson, V.C.; Kimberley, B.S.; Maddox, P.J.; McTavish, S.J.; Solan, G.A.; White, A.J.P.; Williams, D.J. Novel olefin polymerization catalysts based on iron and cobalt. Chem. Commun. 1998, 849–850. [Google Scholar] [CrossRef]
- Small, B.L.; Brookhart, M.; Bennett, A.M.A. Highly active iron and cobalt catalysts for the polymerization of ethylene. J. Am. Chem. Soc. 1998, 120, 4049–4050. [Google Scholar] [CrossRef]
- Ricci, G. Polymerization of 1,3-dienes with iron complexes based catalysts Influence of the ligand on catalyst activity and stereospecificity. J. Mol. Catal. A Chem. 2003, 204–205, 287–293. [Google Scholar] [CrossRef]
- Ricci, G.; Motta, T.; Boglia, A.; Alberti, E.; Zetta, L.; Bertini, F.; Arosio, P.; Famulari, A.; Meille, S.V. Synthesis, characterization, and crystalline structure of syndiotactic 1,2-polypentadiene: The trans polymer. Macromolecules 2005, 38, 8345–8352. [Google Scholar] [CrossRef]
- Ricci, G.; Boglia, A.; Motta, T.; Bertini, F.; Boccia, A.C.; Zetta, L.; Alberti, E.; Famulari, A.; Arosio, P.; Meille, S.V. Synthesis and structural characterization of syndiotactictrans-1,2 and cis-1,2 polyhexadienes. J. Polym. Sci. A Polym. Chem. 2007, 45, 5339–5353. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Bertini, F.; Boccia, A.C.; Zetta, L. Synthesis and characterization of isotactic 1,2-poly(E-3-methyl-1,3-pentadiene). Some remarks about the influence of monomer structure on polymerization stereoselectivity. Macromolecules 2009, 42, 3048–3056. [Google Scholar] [CrossRef]
- Ricci, G.; Leone, G.; Boglia, A.; Boccia, A.C.; Zetta, L. cis-1,4-alt-3,4 polyisoprene: Synthesis and characterization. Macromolecules 2009, 42, 9263–9267. [Google Scholar] [CrossRef]
- He, A.H.; Wang, G.; Zhao, W.Z.; Jiang, X.B.; Yao, W.; Sun, W.H. High cis-1,4 polyisoprene or cis-1,4/3,4 binary polyisoprene synthesized using 2-(benzimidazolyl)-6-(1-(arylimino)ethyl)pyridine cobalt(II) dichlorides. Polym. Int. 2013, 62, 1758–1766. [Google Scholar] [CrossRef]
- Takano, S.; Takeuchi, D.; Osakada, K.; Akamatsu, N.; Shishido, A. Dipalladium catalyst for olefin polymerization: Introduction of acrylate units into the main chain of branched polyethylene. Angew. Chem. Int. Ed. 2014, 53, 9246–9250. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.Y.; Sui, X.L.; Chen, C.L. Highly robust palladium(II) alpha-diimine catalysts for slow-chain-walking polymerization of ethylene and copolymerization with methyl acrylate. Angew. Chem. Int. Ed. 2015, 54, 9948–9953. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Dai, S.Y.; Sui, X.L.; Chen, C.L. Palladium and nickel catalyzed chain walking olefin polymerization and copolymerization. ACS Catal. 2015, 6, 428–441. [Google Scholar] [CrossRef]
- Nakamura, A.; Ito, S.; Nozaki, K. Coordination-insertion copolymerization of fundamental polar monomers. Chem. Rev. 2009, 109, 5215–5244. [Google Scholar] [CrossRef] [PubMed]
- Chen, E.Y.X. Coordination polymerization of polar vinyl monomers by single-site metal catalysts. Chem. Rev. 2009, 109, 5157–5214. [Google Scholar] [CrossRef] [PubMed]
- Raynaud, J.; Wu, J.Y.; Ritter, T. Iron-catalyzed polymerization of isoprene and other 1,3-dienes. Angew. Chem. Int. Ed. 2012, 51, 11805–11808. [Google Scholar] [CrossRef] [PubMed]
- Guo, L.H.; Jing, X.Y.; Xiong, S.Y.; Liu, W.J.; Liu, Y.L.; Liu, Z.; Chen, C.L. Influences of alkyl and aryl substituents on iminopyridine Fe(II)- and Co(II)-catalyzed isoprene polymerization. Polymers 2016, 8, 389. [Google Scholar] [CrossRef]
- Huang, Z.F.; Song, K.M.; Liu, F.S.; Long, J.M.; Hu, H.; Gao, H.Y.; Wu, Q. Synthesis and characterization of a series of 2-aminopyridine nickel(II) complexes and their catalytic properties toward ethylene polymerization. J. Polym. Sci. A Polym. Chem. 2008, 46, 1618–1628. [Google Scholar] [CrossRef]
- Zai, S.B.; Gao, H.Y.; Huang, Z.F.; Hu, H.B.; Wu, H.; Wu, Q. Substituent effects of pyridine-amine nickel catalyst precursors on ethylene polymerization. ACS Catal. 2012, 2, 433–440. [Google Scholar] [CrossRef]
- Hilt, G.; Janikowski, J.; Schwarzer, M.; Burghaus, O.; Sakow, D.; Bröring, M.; Drüschler, M.; Huber, B.; Roling, B.; Harms, K.; et al. Studies of electronic effects of modified pyridine-imine ligands utilized in cobalt-catalyzed meta-selective Diels–Alder reactions. J. Organomet. Chem. 2014, 749, 219–223. [Google Scholar] [CrossRef]
- Iovel, I.; Golomba, L.; Belyakov, S.; Kemme, A.; Lukevics, E. Addition of Me3SiCN to trifluoromethyl derivates of N-(pyridylmethylidene) anilines catalyzed by Lewis acids. Appl. Organomet. Chem. 2001, 15, 733–743. [Google Scholar] [CrossRef]
- Diez, V.; Cuevas, J.V.; Garcia-Herbosa, G.; Aullon, G.; Charmant, J.P.H.; Carbayo, A.; Munoz, A. 1H NMR direct observation of enantiomeric exchange in palladium(II) and platinum(II) complexes containing N,N′-bidentate aryl-pyridin-2-ylmethyl-amine ligands. Inorg. Chem. 2007, 46, 568–577. [Google Scholar] [CrossRef] [PubMed]
- Dai, Q.Q.; Jia, X.Y.; Yang, F.; Bai, C.X.; Hu, Y.M.; Zhang, X.Q. Iminopyridine-based cobalt(II) and nickel(II) complexes: Synthesis, characterization, and their catalytic behaviors for 1,3-butadiene polymerization. Polymers 2016, 8, 12. [Google Scholar] [CrossRef]
- Gong, D.; Jia, W.; Chen, T.; Huang, K.-W. Polymerization of 1,3-butadiene catalyzed by pincer cobalt(II) complexes derived from 2-(1-arylimino)-6-(pyrazol-1-yl)pyridine ligands. Appl. Catal. A Gen. 2013, 464–465, 35–42. [Google Scholar] [CrossRef]
- Gong, D.; Wang, B.; Cai, H.; Zhang, X.; Jiang, L. Synthesis, characterization and butadiene polymerization studies of cobalt(II) complexes bearing bisiminopyridine ligand. J. Organomet. Chem. 2011, 696, 1584–1590. [Google Scholar] [CrossRef]
- Nobbs, J.D.; Tomov, A.K.; Cariou, R.; Gibson, V.C.; White, A.J.; Britovsek, G.J. Thio-Pybox and Thio-Phebox complexes of chromium, iron, cobalt and nickel and their application in ethylene and butadiene polymerisation catalysis. Dalton Trans. 2012, 41, 5949–5964. [Google Scholar] [CrossRef] [PubMed]
- Karakida, K.; Fukuyama, T.; Kuchitsu, K. Molecular-structures of hydrogen-cyanide and acetonitrile as studied by gas electron-diffraction. Bull. Chem. Soc. Jpn. 1974, 47, 299–304. [Google Scholar] [CrossRef]
- Fliedel, C.; Rosa, V.; Vileno, B.; Parizel, N.; Choua, S.; Gourlaouen, C.; Rosa, P.; Turek, P.; Braunstein, P. Zwitterionic cobalt complexes with bis(diphenylphosphino)(N-thioether)amine assembling ligands: Structural, EPR, magnetic, and computational studies. Inorg. Chem. 2016, 55, 4183–4198. [Google Scholar] [CrossRef] [PubMed]
- Jie, S.; Ai, P.; Li, B.G. Highly active and stereospecific polymerization of 1,3-butadiene catalyzed by dinuclear cobalt(II) complexes bearing 3-aryliminomethyl-2-hydroxybenzaldehydes. Dalton Trans. 2011, 40, 10975–10982. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.-M.; Lin, Q.; Wei, T.-B.; Zhang, D.-H.; Jie, S.-Y. Synthesis, structure and ethylene oligomerization behavior of non-symmetric bidentate neutral arylnickel(II) phosphine complexes. Inorg. Chim. Acta 2005, 358, 4423–4430. [Google Scholar] [CrossRef]
- Du, J.L.; Han, L.Q.; Cui, Y.; Li, J.T.; Li, Y.; Sun, W.H. Synthesis, characterization, and ethylene oligomerization of 2,6-bis(imino)phenoxy cobalt complexes. Aust. J. Chem. 2003, 56, 703–706. [Google Scholar] [CrossRef]
- Aggarwal, R.P.; Connelly, N.G.; Crespo, M.C.; Dunne, B.J.; Hopkins, P.M.; Orpen, A.G. Oxidatively induced alkyne rotation in dicobalt complexes-structural tests of molecular-orbital theory. J. Chem. Soc. Dalton Trans. 1992, 655–662. [Google Scholar] [CrossRef]
- Cotton, F.A.; Daniels, L.M.; Feng, X.J.; Maloney, D.J.; Matonic, J.H.; Murillo, C.A. The use of CoCl2(amidine)(2) compounds in the synthesis of tetragonal lantern dicobalt compounds: Synthesis, structures and theoretical studies of Co-2(DPhF)(4) and the oxidized species Co-2(DPhBz)(4) (+) (DPhF = N,N′-diphenylformamidinate, DPhBz = N,N′-diphenylbenzamidinate) 4. Inorg. Chim. Acta 1997, 256, 291–301. [Google Scholar]
- Gaylord, N.G.; Švestka, M. Cyclo- and Cyclized Diene Polymers. XXI. Polymerization of Isoprene by Stable Carbonium Ion Salt Catalysts. J. Macromol. Sci. Part A Chem. 1969, 3, 897–909. [Google Scholar] [CrossRef]
- Ouardad, S.; Deffieux, A.; Peruch, F. Polyisoprene synthesized via cationic polymerization: State of the art. Pure Appl. Chem. 2012, 84, 2065–2080. [Google Scholar] [CrossRef]
- Costabile, C.; Milano, G.; Cavallo, L.; Guerra, G. Stereoselectivity and chemoselectivity in Ziegler-Natta polymerizations of conjugated dienes. 1. monomers with low-energy s-cis-η4 coordination. Macromolecules 2001, 34, 7952–7960. [Google Scholar] [CrossRef]
- Li, X.F.; Nishiura, M.; Hu, L.H.; Mori, K.; Hou, Z.M. Alternating and random copolymerization of isoprene and ethylene catalyzed by cationic half-sandwich scandium alkyls. J. Am. Chem. Soc. 2009, 131, 13870–13882. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.H.; Luo, Y.; Zhou, G.L.; Wang, X.B.; Yu, X.R.; Hou, Z.M.; Qu, J.P. Theoretical mechanistic studies on the trans-1,4-specific polymerization of isoprene catalyzed by a cationic La–Al binuclear complex. Macromolecules 2014, 47, 4596–4606. [Google Scholar] [CrossRef]
- Chandran, D.; Kwak, C.H.; Ha, C.-S.; Kim, I. Polymerization of 1,3-butadiene by bis(salicylaldiminate)cobalt(II) catalysts combined with organoaluminium cocatalysts. Catal. Today 2008, 131, 505–512. [Google Scholar] [CrossRef]



| Entry | Complex | Yield (%) | Microstructure b (%) | Mnc (×10−4) | PDI c | Activity d | ||
|---|---|---|---|---|---|---|---|---|
| cis-1,4 | trans-1,4 | 3,4 | ||||||
| 1 | 1a | >99.0 | 54 | - | 46 | 9.1 | 4.3 | 12.2 |
| 2 | 2a | 85.3 | 54 | - | 46 | 9.7 | 3.5 | 7.2 |
| 3 | 3a | 32.7 | 56 | - | 44 | 19.0 | 2.1 | 2.8 |
| 4 | 4a | 10.9 | - | 90 | 10 | 62.7 | 2.1 | 0.9 |
| 0.4 | 1.7 | |||||||
| 5 | 5a | 21.1 | 65 | - | 35 | 6.1 | 1.5 | 1.8 |
| Entry | Complex | Yield (%) | Microstructure b (%) | Mnc (×10−4) | PDI c | Activity d | |
|---|---|---|---|---|---|---|---|
| cis-1,4 | 3,4 | ||||||
| 1 | 1b | 60.2 | 73 | 27 | 14.0 | 1.8 | 2.6 |
| 2 | 2b | 46.3 | 72 | 28 | 10.5 | 2.2 | 2.0 |
| 3 | 3b | 29.1 | 71 | 29 | 8.0 | 3.0 | 1.2 |
| 4 | 4b | 58.1 | 68 | 32 | 5.3 | 2.5 | 2.5 |
| 5 | 5b | 21.3 | 73 | 27 | 13.9 | 2.1 | 0.9 |
| Entry | Complex | Yield (%) | Microstructure b (%) | Mnc (×10−3) | PDI c | Activity d | |
|---|---|---|---|---|---|---|---|
| trans-1,4 | 3,4 | ||||||
| 1 | 1a | 52.8 | 95 | 5 | 1.4 | 1.7 | 2.2 |
| 2 | 2a | 76.3 | 98 | 2 | 1.5 | 2.1 | 3.2 |
| 3 | 3a | 64.8 | 96 | 4 | 1.6 | 2.2 | 2.8 |
| 4 | 4a | 30.2 | 98 | 2 | 1.5 | 1.8 | 1.3 |
| 5 | 5a | trace | - | - | - | - | - |
| 6 | 1b | 19.3 | 96 | 4 | 1.6 | 1.5 | 0.8 |
| 7 | 2b | 39.7 | 98 | 2 | 1.4 | 1.7 | 1.7 |
| 8 | 3b | 71.8 | 95 | 5 | 1.4 | 1.9 | 3.1 |
| 9 | 4b | 19.6 | 98 | 2 | 1.6 | 1.5 | 0.8 |
| 10 | 5b | 32.2 | 98 | 2 | 1.2 | 1.6 | 1.4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, G.; Zhang, X.; Zhao, M.; Wang, L.; Jing, C.; Wang, P.; Wang, X.; Wang, Q. Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers 2018, 10, 934. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090934
Zhu G, Zhang X, Zhao M, Wang L, Jing C, Wang P, Wang X, Wang Q. Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization. Polymers. 2018; 10(9):934. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090934
Chicago/Turabian StyleZhu, Guangqian, Xianhui Zhang, Mengmeng Zhao, Liang Wang, Chuyang Jing, Peng Wang, Xiaowu Wang, and Qinggang Wang. 2018. "Influences of Fluorine Substituents on Iminopyridine Fe(II)- and Co(II)-Catalyzed Isoprene Polymerization" Polymers 10, no. 9: 934. https://0-doi-org.brum.beds.ac.uk/10.3390/polym10090934