Study on the Printability through Digital Light Processing Technique of Ionic Liquids for CO2 Capture
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Formulation Preparation and Printing
2.3. Characterization
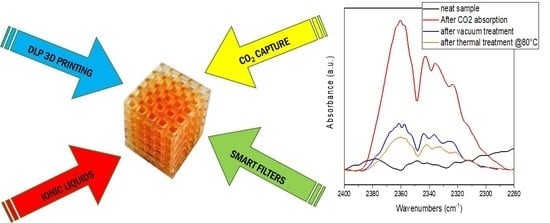
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Figueres, C.; Le Quéré, C.; Mahindra, A.; Bäte, O.; Whiteman, G.; Peters, G.; Guan, D. Emissions are still rising: Ramp up the cuts. Nature 2018, 564, 27–30. [Google Scholar] [CrossRef]
- Jacobson, M.Z. Air Pollution and Global Warming: History, Science, and Solutions, 2nd ed.; Cambridge University Press: Cambridge, UK, 2012. [Google Scholar]
- Hoffmann, M.J. Global climate change. In The Handbook of Global Climate and Environment Policy; Wiley-Blackwell: Oxford, UK, 2013; pp. 3–18. [Google Scholar]
- Araújo, O.D.Q.F.; de Medeiros, J.L. Carbon capture and storage technologies: Present scenario and drivers of innovation. Curr. Opin. Chem. Eng. 2017, 17, 22–34. [Google Scholar] [CrossRef]
- Theo, W.L.; Lim, J.S.; Hashim, H.; Mustaffa, A.A.; Ho, W.S. Review of pre-combustion capture and ionic liquid in carbon capture and storage. Appl. Energy 2016, 183, 1633–1663. [Google Scholar] [CrossRef]
- Jansen, D.; Gazzani, M.; Manzolini, G.; Dijk, E.V.; Carbo, M. Pre-combustion CO2 capture. Int. J. Greenh. Gas Control 2015, 40, 167–187. [Google Scholar] [CrossRef]
- Hadjipaschalis, I.; Kourtis, G.; Poullikkas, A. Assessment of oxyfuel power generation technologies. Renew. Sustain. Energy Rev. 2009, 13, 2637–2644. [Google Scholar] [CrossRef]
- Wang, C.A.; Zhang, X.; Liu, Y.; Che, D. Pyrolysis and combustion characteristics of coals in oxyfuel combustion. Appl. Energy 2012, 97, 264–273. [Google Scholar] [CrossRef]
- Machida, H.; Ando, R.; Esaki, T.; Yamaguchi, T.; Norinaga, K. Modelling of CO2 solubility in phase separation solvent composed of amine/ether/water system for CO2 capture. J. Mol. Liq. 2019, 292, 111411. [Google Scholar] [CrossRef]
- Kussainova, D.; Shah, D. Monoethanolamine based DESs for CO2 absorption: Insights from molecular dynamics simulations. Sep. Purif. Technol. 2020, 231, 115931. [Google Scholar] [CrossRef]
- Meisen, A.; Shuai, X. Research and development issues in CO2 capture. Energy Convers. Manag. 1997, 38, S37–S42. [Google Scholar] [CrossRef]
- Andirova, D.; Cogswell, C.F.; Lei, Y.; Choi, S. Effect of the structural constituents of metal organic frameworks on carbon dioxide capture. Microporous Mesoporous Mater. 2016, 219, 276–305. [Google Scholar] [CrossRef]
- Nandasiri, M.I.; Jambovane, S.R.; McGrail, B.P.; Schaef, H.T.; Nune, S.K. Adsorption, separation, and catalytic properties of densified metal-organic frameworks. Coord. Chem. Rev. 2016, 311, 38–52. [Google Scholar] [CrossRef]
- Venna, S.R.; Carreon, M.A. Metal organic framework membranes for carbon dioxide separation. Chem. Eng. Sci. 2015, 124, 3–19. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, Q.; Zhang, G. Metal−organic framework composite membranes: Synthesis and separation applications. Chem. Eng. Sci. 2015, 135, 232–257. [Google Scholar] [CrossRef]
- Heo, Y.J.; Seong, D.B.; Park, S.J. Synthesis of polyethylenimine-impregnated titanate nanotubes for CO2 capture: Influence of porosity and nitrogen content on amine-modified adsorbents. J. CO2 Util. 2019, 34, 472–478. [Google Scholar] [CrossRef]
- Zhao, P.; Zhang, G.; Xu, Y.; Lv, Y. Amine functionalized hierarchical bimodal mesoporous silicas as a promising nanocomposite for highly efficient CO2 capture. J. CO2 Util. 2019, 34, 543–557. [Google Scholar] [CrossRef]
- Stefanelli, E.; Puccini, M.; Vitolo, S.; Seggiani, M. CO2 sorption kinetic study and modeling on doped-Li4SiO4 under different temperatures and CO2 partial pressures. Chem. Eng. J. 2020, 379, 122307. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Hu, Y.; Sun, J.; Tong, X.; Li, Q.; Zhou, Z. Novel low cost Li4SiO4-based sorbent with naturally occurring wollastonite as Si-source for cyclic CO2 capture. Chem. Eng. J. 2019, 374, 328–337. [Google Scholar] [CrossRef]
- Halder, K.; Khan, M.M.; Grünauer, J.; Shishatskiy, S.; Abetz, C.; Filiz, V.; Abetz, V. Blend membranes of ionic liquid and polymers of intrinsic microporosity with improved gas separation characteristics. J. Membr. Sci. 2017, 539, 368–382. [Google Scholar] [CrossRef]
- Marsh, K.N.; Boxall, J.A.; Lichtenthaler, R. Room temperature ionic liquids and their mixtures—A review. Fluid Phase Equilibria 2004, 219, 93–98. [Google Scholar] [CrossRef]
- Zeng, S.; Zhang, X.; Bai, L.; Wang, H.; Wang, J.; Bao, D.; Li, M.; Liu, X.; Zhang, S. Ionic-Liquid-Based CO2 Capture Systems: Structure, Interaction and Process. Chem. Rev. 2017, 117, 9625–9673. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A. Characterization and comparison of the CO2 absorption performance into single and blended alkanolamines in a packed column. Ind. Eng. Chem. Res. 2004, 43, 2228–2237. [Google Scholar] [CrossRef]
- Sistla, Y.S.; Khanna, A. CO2 absorption studies in amino acid-anion based ionic liquids. Chem. Eng. J. 2015, 273, 268–276. [Google Scholar] [CrossRef]
- Makino, T.; Kanakubo, M. CO2 absorption property of ionic liquid and CO2 permselectivity for ionic liquid membrane. J. Jpn. Pet. Inst. 2016, 59, 109–117. [Google Scholar] [CrossRef]
- Lozano, L.J.; Godínez, C.; de los Ríos, A.P.; Hernández-Fernández, F.J.; Sánchez-Segado, S.; Alguacil, F.J. Recent advances in supported ionic liquid membrane technology. J. Membr. Sci. 2011, 376, 1–14. [Google Scholar] [CrossRef]
- Malik, M.A.; Hashim, M.A.; Nabi, F. Ionic liquids in supported liquid membrane technology. Chem. Eng. J. 2011, 171, 242–254. [Google Scholar] [CrossRef]
- Parhi, P.K. Supported liquid membrane principle and its practices: A short review. J. Chem. 2013. [Google Scholar] [CrossRef]
- Dai, Z.; Noble, R.D.; Gin, D.L.; Zhang, X.; Deng, L. Combination of ionic liquids with membrane technology: A new approach for CO2 separation. J. Membr. Sci. 2016, 497, 1–20. [Google Scholar] [CrossRef]
- Zulfiqar, S.; Sarwar, M.I.; Mecerreyes, D. Polymeric ionic liquids for CO2 capture and separation: Potential, progress and challenges. Polym. Chem. 2015, 6, 6435–6451. [Google Scholar] [CrossRef]
- Tang, J.; Sun, W.; Tang, H.; Radosz, M.; Shen, Y. Enhanced CO2 Absorption of Poly(ionic liquid)s. Macromolecules 2005, 38, 2037–2039. [Google Scholar] [CrossRef]
- Tang, J.; Shen, Y.; Radosz, M.; Sun, W. Isothermal Carbon Dioxide Sorption in Poly(ionic liquid)s. Ind. Eng. Chem. Res. 2009, 48, 9113–9118. [Google Scholar] [CrossRef]
- Tomé, L.C.; Gouveia, A.S.L.; Freire, C.S.R.; Mecerreyes, D.; Marrucho, I.M. Polymeric ionic liquid-based membranes: Influence of polycation variation on gas transport and CO2 selectivity properties. J. Membr. Sci. 2015, 486, 40–48. [Google Scholar] [CrossRef] [Green Version]
- Bara, J.E.; Lessmann, S.; Gabriel, C.J.; Hatakeyama, E.S.; Noble, R.D.; Gin, D.L. Synthesis and Performance of Polymerizable Room-Temperature Ionic Liquids as Gas Separation Membranes. Ind. Eng. Chem. Res. 2007, 46, 5397–5404. [Google Scholar] [CrossRef]
- Zhou, X.; Weber, J.; Yuan, J. Poly(ionic liquid)s: Platform for CO2 capture and catalysis. Curr. Opin. Green Sustain. Chem. 2019, 16, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Steldinger, H.; Esposito, A.; Brunnengräber, K.; Gläsel, J.; Etzold, B.J.M. Activated Carbon in the Third Dimension—3D Printing of a Tuned Porous Carbon. Adv. Sci. 2019, 6, 1901340. [Google Scholar] [CrossRef] [Green Version]
- Chiappone, A.; Fantino, E.; Roppolo, I.; Lorusso, M.; Manfredi, D.; Fino, P.; Pirri, C.F.; Calignano, F. 3D Printed PEG-Based Hybrid Nanocomposites Obtained by Sol–Gel Technique. ACS Appl. Mater. Interfaces 2016, 8, 5627–5633. [Google Scholar] [CrossRef]
- Farahani, R.D.; Dubé, M.; Therriault, D. Three-Dimensional Printing of Multifunctional Nanocomposites: Manufacturing Techniques and Applications. Adv. Mater. 2016, 28, 5794–5821. [Google Scholar] [CrossRef]
- Emon, M.O.F.; Alkadi, F.; Philip, D.G.; Kim, D.-H.; Lee, K.-C.; Choi, J.-W. Multi-material 3D printing of a soft pressure sensor. Addit. Manuf. 2019, 28, 629–638. [Google Scholar] [CrossRef]
- Crump, M.R.; Gong, A.T.; Chai, D.; Bidinger, S.L.; Pavinatto, F.J.; Reihsen, T.E.; Sweet, R.M.; Mackenzie, J.D. Monolithic 3D printing of embeddable and highly stretchable strain sensors using conductive ionogels. Nanotechnology 2019, 30, 364002. [Google Scholar] [CrossRef]
- Wong, J.; Gong, A.T.; Defnet, P.A.; Meabe, L.; Beauchamp, B.; Sweet, R.M.; Sardon, H.; Cobb, C.L.; Nelson, A. 3D Printing Ionogel Auxetic Frameworks for Stretchable Sensors. Adv. Mater. Technol. 2019, 4, 1900452. [Google Scholar] [CrossRef]
- Chen, G.; Chen, N.; Wang, Q. Preparation of poly (vinyl alcohol)/ionic liquid composites with improved processability and electrical conductivity for fused deposition modeling. Mater. Des. 2018, 157, 273–283. [Google Scholar] [CrossRef]
- Schultz, A.R.; Lambert, P.M.; Chartrain, N.A.; Ruohoniemi, D.M.; Zhang, Z.; Jangu, C.; Zhang, M.; Williams, C.B.; Long, T.E. 3D Printing Phosphonium Ionic Liquid Networks with Mask Projection Microstereolithography. ACS Macro Lett. 2014, 3, 1205–1209. [Google Scholar] [CrossRef]
- Lee, J.; Faruk Emon, M.O.; Vatani, M.; Choi, J.W. Effect of degree of crosslinking and polymerization of 3D printable polymer/ionic liquid composites on performance of stretchable piezoresistive sensors. Smart Mater. Struct. 2017, 26, 035043. [Google Scholar] [CrossRef]
- Wales, D.J.; Cao, Q.; Kastner, K.; Karjalainen, E.; Newton, G.N.; Sans, V. 3D-Printable Photochromic Molecular Materials for Reversible Information Storage. Adv. Mater. 2018, 30, 1800159. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Yan, Y.; Zhang, L.; Qin, Z.; Tao, S. Enhanced Mass Transfer and Improved Catalyst Recovery in a Stirred Reactor by Polymeric Ionic Liquids Modified 3D Printed Devices. Adv. Mater. Technol. 2019, 4, 1800515. [Google Scholar] [CrossRef]
- Ding, H.; Chang, R.C. Printability study of bioprinted tubular structures using liquid hydrogel precursors in a support bath. Appl. Sci. (Switzerland) 2018, 8, 403. [Google Scholar] [CrossRef] [Green Version]
- Melchels, F.P.W.; Feijen, J.; Grijpma, D.W. A review on stereolithography and its applications in biomedical engineering. Biomaterials 2010, 31, 6121–6130. [Google Scholar] [CrossRef] [Green Version]
- Frascella, F.; González, G.; Bosch, P.; Angelini, A.; Chiappone, A.; Sangermano, M.; Pirri, C.F.; Roppolo, I. Three-Dimensional Printed Photoluminescent Polymeric Waveguides. ACS Appl. Mater. Interfaces 2018, 10, 39319–39326. [Google Scholar] [CrossRef]
- Roppolo, I.; Chiappone, A.; Angelini, A.; Stassi, S.; Frascella, F.; Pirri, C.; Ricciardi, C.; Descrovi, E. 3D printable light-responsive polymers. Mater. Horiz. 2017, 4, 396–401. [Google Scholar] [CrossRef]
- Ligon, S.C.; Liska, R.; Stampfl, J.; Gurr, M.; Mülhaupt, R. Polymers for 3D Printing and Customized Additive Manufacturing. Chem. Rev. 2017, 117, 10212–10290. [Google Scholar] [CrossRef] [Green Version]
- Nulwala, H.; Mirjafari, A.; Zhou, X. Ionic liquids and poly(ionic liquid)s for 3D printing—A focused mini-review. Eur. Polym. J. 2018, 108, 390–398. [Google Scholar] [CrossRef]
- Jakubiak, J.; Allonas, X.; Fouassier, J.; Sionkowska, A.; Andrzejewska, E.; Linden, L.; Rabek, J. Camphorquinone–amines photoinitating systems for the initiation of free radical polymerization. Polymer 2003, 44, 5219–5226. [Google Scholar] [CrossRef]
- Litt, M.; Eirich, F.R. Polymerization of allyl acetate. J. Polym. Sci. 1960, 45, 379–396. [Google Scholar] [CrossRef]
- Matsumoto, A.; Kumagai, T.; Aota, H.; Kawasaki, H.; Arakawa, R. Reassessment of Free-Radical Polymerization Mechanism of Allyl Acetate Based on End-Group Determination of Resulting Oligomers by MALDI-TOF-MS Spectrometry. Polym. J. 2009, 41, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Chiappone, A.; Jeremias, S.; Bongiovanni, R.; Schönhoff, M. NMR study of photo-crosslinked solid polymer electrolytes: The influence of monofunctional oligoethers. J. Polym. Sci. Part B Polym. Phys. 2013, 51, 1571–1580. [Google Scholar] [CrossRef]
- Gillono, M.; Roppolo, I.; Frascella, F.; Scaltrito, L.; Pirri, C.F.; Chiappone, A. CO2 permeability control in 3D printed light responsive structures. Appl. Mater. Today 2019, 100470. [Google Scholar] [CrossRef]
- Yamazaki, T.; Katoh, M.; Ozawa, S.; Ogino, Y. Adsorption of CO2 over univalent cation-exchanged ZSM-5 zeolites. Mol. Phys. 1993, 80, 313–324. [Google Scholar] [CrossRef]
- Veyrié, D.; Lellouchi, D.; Roux, J.; Pressecq, F.; Tetelin, A.; Pellet, C. FTIR spectroscopy for the hermeticity assessment of micro-cavities. Microelectron. Reliab. 2005, 45, 1764–1769. [Google Scholar] [CrossRef]
- Al-Ketan, O.; Rezgui, R.; Rowshan, R.; Du, H.; Fang, N.X.; Abu Al-Rub, R.K. Microarchitected stretching-dominated mechanical metamaterials with minimal surface topologies. Adv. Eng. Mater. 2018, 20, 1800029. [Google Scholar] [CrossRef]
- Yoo, D.-J. Advanced porous scaffold design using multi-void triply periodic minimal surface models with high surface area to volume ratios. Int. J. Precis. Eng. Manuf. 2014, 15, 1657–1666. [Google Scholar] [CrossRef]




Butyl-3-Methylimidazolium tetrafluoroborate Bmim[BF4]  | N,N,N,N-ButyldimethylMethacryloyloxyethyl ammonium bis(trifluoromethylsulfonyl)imideC4NMA,11[Tf2N] |
1-Butyl-3-Methylimidazolium bis (trifluorometilsulfonyl)imide Bmim[Tf2N] | 1-Allyl-3-Methylimidazolium bis (trifluoromethylsulfonyl)imide Amim[Tf2N] |
1-Butyl-3-Methylimidazolium acetate Bmim[ac] | 1,4-Butandiyl-3,3′-bis-1-vinylimidazoliumbis (trifluoromethylsulfonyl)imide Bvim[Tf2N] |
| Sample | Layer Exposure Time (s) | |
|---|---|---|
| Membrane | Cubic Filter-Like | |
| PEGDA | 1 | 2 |
| P_Bmim[BF4] | 1 | 1.7 |
| P_Bmim[Tf2N] | 1 | 2 |
| P_C4NMA,11[Tf2N] | 1 | 1.8 |
| P_Amim[Tf2N] | 2 | Not obtained |
| P_Bvim[Tf2N] | 1 | 1.7 |
| Sample | Ionic Liquid Concentration (wt %) | Weight Variation (wt %) | Tg (DSC; °C) | Tg (DMTA; °C) | υ (mmol/cm3) |
|---|---|---|---|---|---|
| PEGDA | 0 | 0 | −19.9 | −17 | 10.8 |
| P_Bmim[BF4] | 9 | 8.5 | −22.6 | −21.3 | 1.3 |
| P_Bmim[Tf2N] | 15.1 | 13.9 | −22.5 | −21.6 | 1.4 |
| P_C4NMA,11[Tf2N] | 17.5 | 0 | −15.1 | −12.8 | 1.6 |
| P_Amim[Tf2N] | 14.7 | 14.2 | −44.8 | Not calculated | - |
| P_Bvim[Tf2N] | 14.7 | 0 | −11.2 | −10 | 12.2 |
| Sample | S | ||
|---|---|---|---|
| PEGDA | 6.73 | 4.71 | 3.17 |
| P_Bmim[BF4] | 11.33 | 2.99 | 3.39 |
| P_Bmim[Tf2N] | 8.65 | 5.26 | 4.55 |
| P_C4NMA,11[Tf2N] | 4.46 | 4.22 | 1.88 |
| P_Amim[Tf2N] | 9.53 | 14.47 | 13.79 |
| P_Bvim[Tf2N] | 8.45 | 3.20 | 2.70 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gillono, M.; Chiappone, A.; Mendola, L.; Gomez Gomez, M.; Scaltrito, L.; Pirri, C.F.; Roppolo, I. Study on the Printability through Digital Light Processing Technique of Ionic Liquids for CO2 Capture. Polymers 2019, 11, 1932. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121932
Gillono M, Chiappone A, Mendola L, Gomez Gomez M, Scaltrito L, Pirri CF, Roppolo I. Study on the Printability through Digital Light Processing Technique of Ionic Liquids for CO2 Capture. Polymers. 2019; 11(12):1932. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121932
Chicago/Turabian StyleGillono, Matteo, Annalisa Chiappone, Lorenzo Mendola, Manuel Gomez Gomez, Luciano Scaltrito, Candido Fabrizio Pirri, and Ignazio Roppolo. 2019. "Study on the Printability through Digital Light Processing Technique of Ionic Liquids for CO2 Capture" Polymers 11, no. 12: 1932. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11121932