Synthesis and Properties of Tung Oil-Based Unsaturated Co-Ester Resins Bearing Steric Hindrance
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Synthesis of TOPERMA
2.3. Synthesis of TO-Based Co-UEs
2.4. Curing of the TO-Based Resins
2.5. Characterization
2.5.1. Gel Permeation Chromatography (GPC)
2.5.2. Nuclear Magnetic Resonance (NMR)
2.5.3. Dynamic Mechanical Analysis (DMA)
2.5.4. Thermogravimetric Analysis (TGA)
2.5.5. Mechanical Properties
2.5.6. Water Absorption
3. Results and Discussion
3.1. Characterization of TO-Based Co-UEs
3.2. Properties of TO-Based Co-UE Resins
3.2.1. Dynamic Mechanical Analysis
3.2.2. Thermogravimetric Analysis
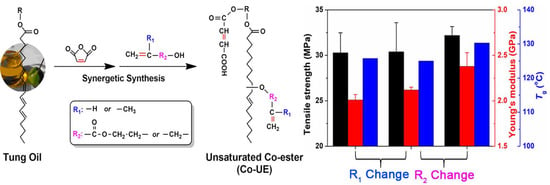
3.2.3. Mechanical Properties
3.2.4. Water Absorption
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kandelbauer, A.; Tondi, G.; Zaske, O.C.; Goodman, S.H. Unsaturated polyesters and vinyl esters. In Handbook of Thermoset Plastics, 3rd ed.; Dodiuk, H., Goodman, S.H., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 111–172. [Google Scholar]
- Barile, C.; Casavola, C.; De Cillis, F. Mechanical comparison of new composite materials for aerospace applications. Compos. Part B-Eng. 2019, 162, 122–128. [Google Scholar]
- Liu, C.G.; Wang, C.N.; Tang, J.J.; Zhang, J.; Shang, Q.Q.; Hu, Y.; Wang, H.X.; Wu, Q.; Zhou, Y.H.; Lei, W.; et al. High-performance biobased unsaturated polyester nanocomposites with very low loadings of graphene. Polymers 2018, 10, 1288. [Google Scholar] [CrossRef] [PubMed]
- Kandola, B.K.; Ebdon, J.R.; Chowdhury, K.P. Flame retardance and physical properties of novel cured blends of unsaturated polyester and furan resins. Polymers 2015, 7, 298–315. [Google Scholar] [CrossRef]
- Raquez, J.M.; Deleglise, M.; Lacrampe, M.F.; Krawczak, P. Thermosetting (bio)materials derived from renewable resources: A critical review. Prog. Polym. Sci. 2010, 35, 487–509. [Google Scholar] [CrossRef]
- Chen, J.Q.; Tang, C.Q.; Yue, Y.Y.; Qiao, W.C.; Hong, J.G.; Kitaoka, T.; Yang, Z. Highly translucent all wood plastics via heterogeneous esterification in ionic liquid/dimethyl sulfoxide. Ind. Crop. Prod. 2017, 108, 286–294. [Google Scholar] [CrossRef]
- Guo, Y.; Chen, J.Q.; Su, M.; Hong, J.G. Bio-based plastics with highly efficient esterification of lignocellulosic biomass in 1-methylimidazole under mild conditions. J. Wood Chem. Technol. 2018, 38, 338–349. [Google Scholar] [CrossRef]
- Feng, Y.C.; Liang, H.Y.; Yang, Z.M.; Yuan, T.; Luo, Y.; Li, P.W.; Yang, Z.H.; Zhang, C.Q. A solvent-free and scalable method to prepare soybean-oil-based polyols by thiol-ene photo-click reaction and biobased polyurethanes therefrom. ACS Sustain. Chem. Eng. 2017, 5, 7365–7373. [Google Scholar]
- Zhang, C.Q.; Garrison, T.F.; Madbouly, S.A.; Kessler, M.R. Recent advances in vegetable oil-based polymers and their composites. Prog. Polym. Sci. 2017, 71, 91–143. [Google Scholar] [CrossRef]
- Chen, J.Q.; Su, M.; Zhang, X.L.; Chen, R.P.; Hong, J.G.; Yang, L.Y.; Yang, Z. The role of cations in homogeneous succinoylation of mulberry wood cellulose in salt-containing solvents under mild conditions. Cellulose 2014, 21, 4081–4091. [Google Scholar] [CrossRef]
- Huang, C.; Dong, H.; Su, Y.; Wu, Y.; Narron, R.; Yong, Q. Synthesis of carbon quantum dot nanoparticles derived from byproducts in bio-refinery process for cell imaging and in vivo bioimaging. Nanomaterials 2019, 9, 387. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Su, Y.; Shi, J.; Yuan, C.; Zhai, S.; Yong, Q. Revealing the effects of centuries of ageing on the chemical structural features of lignin in archaeological fir woods. New J. Chem. 2019, 43, 3520–3528. [Google Scholar] [CrossRef]
- Wool, R.P.; Sun, X.S. Bio-Based Polymers and Composites; Elsevier: Amesterdam, The Netherlands, 2005. [Google Scholar]
- Liu, W.; Fei, M.-E.; Ban, Y.; Jia, A.; Qiu, R. Preparation and evaluation of green composites from microcrystalline cellulose and a soybean-oil derivative. Polymers 2017, 9, 541. [Google Scholar] [CrossRef] [PubMed]
- Costa, C.; Fonseca, A.C.; Moniz, J.; Godinho, M.; Serra, A.C.; Coelho, J.F.J. Soybean and coconut oil based unsaturated polyester resins: Thermomechanical characterization. Ind. Crop Prod. 2016, 85, 403–411. [Google Scholar] [CrossRef]
- Qin, Y.; Jia, J.; Zhao, L.; Huang, Z.; Zhao, S.; Zhang, G.; Dai, B. Synthesis and characterization of soybean oil based unsaturated polyester resin. In Biotechnology, Chemical and Materials Engineering, Pts 1-3; Chen, R., Sung, W.P., Eds.; Trans Tech Publications Ltd.: Zurich, Switzerland, 2012; Volumes 393–395, pp. 349–353. [Google Scholar]
- Khot, S.N.; Lascala, J.J.; Can, E.; Morye, S.S.; Williams, G.I.; Palmese, G.R.; Kusefoglu, S.H.; Wool, R.P. Development and application of triglyceride-based polymers and composites. J. Appl. Polym. Sci. 2001, 82, 703–723. [Google Scholar] [CrossRef]
- Can, E.; Kusefoglu, S.; Wool, R.P. Rigid, thermosetting liquid molding resins from renewable resources. I. Synthesis and polymerization of soy oil monoglyceride maleates. J. Appl. Polym. Sci. 2001, 81, 69–77. [Google Scholar] [CrossRef]
- Can, E.; Wool, R.P.; Kusefoglu, S. Soybean and castor oil based monomers: Synthesis and copolymerization with styrene. J. Appl. Polym. Sci. 2006, 102, 2433–2447. [Google Scholar] [CrossRef]
- Eren, T.; Kusefoglu, S.H. Synthesis and polymerization of the acrylamide derivatives of fatty compounds. J. Appl. Polym. Sci. 2005, 97, 2264–2272. [Google Scholar] [CrossRef]
- Eren, T.; Kusefoglu, S.H. Synthesis and polymerization of the bromoacrylated plant oil triglycerides to rigid, flame-retardant polymers. J. Appl. Polym. Sci. 2004, 91, 2700–2710. [Google Scholar] [CrossRef]
- Lu, J.; Khot, S.; Wool, R.P. New sheet molding compound resins from soybean oil. I. Synthesis and characterization. Polymer 2005, 46, 71–80. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Rios, L.A.; Rivas, B.L. Synthesis and copolymerization of thermosetting resins obtained from vegetable oils and biodiesel-derived crude glycerol. Eur. Polym. J. 2015, 67, 428–438. [Google Scholar] [CrossRef]
- Zhang, P.; Zhang, J.W. One-step acrylation of soybean oil (so) for the preparation of so-based macromonomers. Green Chem. 2013, 15, 641–645. [Google Scholar] [CrossRef]
- Liu, C.G.; Dai, Y.; Hu, Y.; Shang, Q.Q.; Feng, G.D.; Zhou, J.; Zhou, Y.H. Highly functional unsaturated ester macromonomer derived from soybean oil: Synthesis and copolymerization with styrene. ACS Sustain. Chem. Eng. 2016, 4, 4208–4216. [Google Scholar] [CrossRef]
- Gomez, C.L.; Echeverri, D.A.; Inciarte, H.C.; Rios, L.A. Efficient processing of bioglycerol to a novel biobased polyunsaturated monomer. J. Chem. Technol. Biotechnol. 2019, 94, 634–640. [Google Scholar] [CrossRef]
- Liu, C.G.; Shang, Q.Q.; Jia, P.Y.; Dai, Y.; Zhou, Y.H.; Liu, Z.S. Tung oil-based unsaturated co-ester macromonomer for thermosetting polymers: Synergetic synthesis and copolymerization with styrene. ACS Sustain. Chem. Eng. 2016, 4, 3437–3449. [Google Scholar] [CrossRef]
- Liu, C.G.; Yang, X.H.; Cui, J.F.; Zhou, Y.H.; Hu, L.H.; Zhang, M.; Liu, H.J. Tung oil based monomer for thermosetting polymers: Synthesis, characterization, and copolymerization with styrene. Bioresources 2012, 7, 447–463. [Google Scholar]
- Liu, C.G.; Zhou, Y.H.; Cheng, R.S. Quantitative characterization of complex formation of a pmma/peg solution by sec-ls. J. Liq. Chromatogr. Relat. Technol. 2013, 36, 2534–2546. [Google Scholar]
- Can, E.; Wool, R.P.; Küsefoğlu, S. Soybean- and castor-oil-based thermosetting polymers: Mechanical properties. J. Appl. Polym. Sci. 2006, 102, 1497–1504. [Google Scholar] [CrossRef]
- Andjelkovic, D.D.; Valverde, M.; Henna, P.; Li, F.; Larock, R.C. Novel thermosets prepared by cationic copolymerization of various vegetable oils—Synthesis and their structure–property relationships. Polymer 2005, 46, 9674–9685. [Google Scholar] [CrossRef]







| Sample ID | Mwa (g/mol) | Mnb (g/mol) | Dc | NC=Cd |
|---|---|---|---|---|
| TOPERMA | 2247 | 1043 | 2.15 | 1.26 |
| TOPERMA-HEA | 3396 | 1406 | 2.42 | 1.62 |
| TOPERMA-HEMA | 3099 | 1298 | 2.39 | 1.56 |
| TOPERMA-MAA | 3120 | 1313 | 2.38 | 1.61 |
| Samples | E′25a (GPa) | Tgb (°C) | νec (103 mol/m3) | T5d (°C) | Tp1e (°C) | Tp2e (°C) | wchar f (%) |
|---|---|---|---|---|---|---|---|
| TOPERMA | 2.05 | 123.1 | 3.44 | 384.9 | 408.0 | 453.8 | 9.04 |
| TOPERMA-HEA | 2.07 | 125.8 | 4.28 | 380.2 | 413.0 | 455.1 | 8.45 |
| TOPERMA-HEMA | 2.11 | 125.0 | 3.75 | 370.8 | 403.8 | 442.7 | 10.2 |
| TOPERMA-MAA | 2.33 | 130.3 | 3.93 | 379.5 | 406.4 | 452.3 | 8.36 |
| Samples | σ a (MPa) | E b (GPa) | ε c (%) |
|---|---|---|---|
| TOPERMA | 27.4 ± 2.2 | 1.91 ± 0.02 | 4.03 ± 0.29 |
| TOPERMA-HEA | 30.3 ± 2.2 | 2.01 ± 0.06 | 3.98 ± 0.11 |
| TOPERMA-HEMA | 30.9 ± 1.7 | 2.12 ± 0.03 | 3.11 ± 0.04 |
| TOPERMA-MAA | 32.2 ± 1.0 | 2.38 ± 0.15 | 3.79 ± 0.25 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, C.; Wu, Q.; An, R.; Shang, Q.; Feng, G.; Hu, Y.; Jia, P.; Zhou, Y.; Lei, W. Synthesis and Properties of Tung Oil-Based Unsaturated Co-Ester Resins Bearing Steric Hindrance. Polymers 2019, 11, 826. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050826
Liu C, Wu Q, An R, Shang Q, Feng G, Hu Y, Jia P, Zhou Y, Lei W. Synthesis and Properties of Tung Oil-Based Unsaturated Co-Ester Resins Bearing Steric Hindrance. Polymers. 2019; 11(5):826. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050826
Chicago/Turabian StyleLiu, Chengguo, Qiong Wu, Rongrong An, Qianqian Shang, Guodong Feng, Yun Hu, Puyou Jia, Yonghong Zhou, and Wen Lei. 2019. "Synthesis and Properties of Tung Oil-Based Unsaturated Co-Ester Resins Bearing Steric Hindrance" Polymers 11, no. 5: 826. https://0-doi-org.brum.beds.ac.uk/10.3390/polym11050826