Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries
Abstract
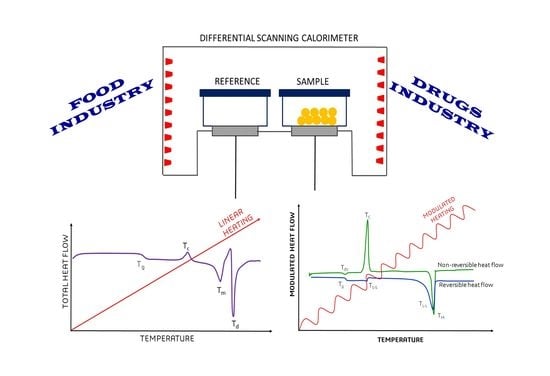
:1. Introduction
1.1. Phase Transitions
1.2. Phase Transition Characterization by Thermal Analysis
2. Modulated Differential Scanning Calorimetry (MDSC)
3. Supercooling Modulated Differential Scanning Calorimetry (SMDSC)
4. Applications of DSC and MDSC in the Industrial Processing and Conservation of Matter
4.1. Food Products
4.2. Pharmaceutical Products
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Langrish, T.; Fletcher, D. Spray drying of food ingredients and applications of CFD in spray drying. Chem. Eng. Process. Process Intensif. 2001, 40, 345–354. [Google Scholar] [CrossRef]
- Roos, Y.H. Phase Transitions in Foods; Academic Press: London, UK, 1995; p. 354. [Google Scholar]
- Rizvi, S.S. Thermodynamic properties of foods in dehydration. In Engineering Properties of Foods; CRC Press: Boca Raton, FL, USA, 2014; pp. 261–348. [Google Scholar]
- Cook, G.; Dickerson, R. Understanding the chemical potential. Am. J. Phys. 1995, 63, 737–742. [Google Scholar] [CrossRef]
- Cahn, J.W. Phase separation by spinodal decomposition in isotropic systems. J. Chem. Phys. 1965, 42, 93–99. [Google Scholar] [CrossRef]
- Job, G.; Herrmann, F. Chemical potential—A quantity in search of recognition. Eur. J. Phys. 2006, 27, 353. [Google Scholar] [CrossRef] [Green Version]
- Bak, P.; Krinsky, S.; Mukamel, D. First-Order Transitions, Symmetry, and the ε Expansion. Phys. Rev. Lett. 1976, 36, 52. [Google Scholar] [CrossRef]
- Liu, Y.; Bhandari, B.; Zhou, W. Glass transition and enthalpy relaxation of amorphous food saccharides: A review. J. Agric. Food Chem. 2006, 54, 5701–5717. [Google Scholar] [CrossRef]
- Rahman, S.; Islam, M.; Mujumdar, A. A study of coupled heat and mass transfer in composite food products during convective drying. Dry. Technol. 2007, 25, 1359–1368. [Google Scholar] [CrossRef]
- Rahman, M.S. Food stability determination by macro–micro region concept in the state diagram and by defining a critical temperature. J. Food Eng. 2010, 99, 402–416. [Google Scholar] [CrossRef]
- Sablani, S.S.; Syamaladevi, R.M.; Swanson, B.G. A review of methods, data and applications of state diagrams of food systems. Food Eng. Rev. 2010, 2, 168–203. [Google Scholar] [CrossRef]
- Kasapis, S. Definition and applications of the network glass transition temperature. Food Hydrocoll. 2006, 20, 218–228. [Google Scholar] [CrossRef]
- Saavedra-Leos, M.; Alvarez-Salas, C.; Esneider-Alcalá, M.; Toxqui-Terán, A.; Pérez-García, S.; Ruiz-Cabrera, M. Towards an improved calorimetric methodology for glass transition temperature determination in amorphous sugars. CyTA J. Food 2012, 10, 258–267. [Google Scholar] [CrossRef]
- Ronkart, S.; Blecker, C.; Fougnies, C.; Van Herck, J.C.; Wouters, J.; Paquot, M. Determination of physical changes of inulin related to sorption isotherms: An X-ray diffraction, modulated differential scanning calorimetry and environmental scanning electron microscopy study. Carbohydr. Polym. 2006, 63, 210–217. [Google Scholar] [CrossRef]
- Baird, J.A.; Taylor, L.S. Evaluation of amorphous solid dispersion properties using thermal analysis techniques. Adv. Drug Deliv. Rev. 2012, 64, 396–421. [Google Scholar] [CrossRef] [PubMed]
- Gunaratne, A.; Hoover, R. Effect of heat–moisture treatment on the structure and physicochemical properties of tuber and root starches. Carbohydr. Polym. 2002, 49, 425–437. [Google Scholar] [CrossRef]
- Wendlandt, W.W. Thermal Methods of Analysis; Wiley-Interscience: New York, NY, USA, 1974. [Google Scholar]
- Haines, P.J. Thermal Methods of Analysis: Principles, Applications and Problems; Springer Science & Business Media: Dordrecht, The Netherlands, 2012. [Google Scholar]
- Gabbott, P. Principles and Applications of Thermal Analysis; John Wiley & Sons: Hoboken, NJ, USA, 2008. [Google Scholar]
- Suriñach, S.; Baró, M.D.; Bordas, A.S.; Clavaguera, N.; Clavaguera-Mora, M.T. La calorimetría diferencial de barrido y su aplicación a la Ciencia de Materiales. Bol. Soc. Española Ceram. Vidr. 1992, 31, 11–17. [Google Scholar]
- Toxqui-Terán, A.; Leyva-Porras, C.; Ruíz-Cabrera, M.; Cruz-Alcantar, P.; Saavedra-Leos, M. Thermal Study of Polyols for the Technological Application as Plasticizers in Food Industry. Polymers 2018, 10, 467. [Google Scholar] [CrossRef] [Green Version]
- Saavedra-Leos, M.; Leyva-Porras, C.; Martínez-Guerra, E.; Pérez-García, S.; Aguilar-Martínez, J.; Álvarez-Salas, C. Physical properties of inulin and inulin–orange juice: Physical characterization and technological application. Carbohydr. Polym. 2014, 105, 10–19. [Google Scholar] [CrossRef]
- Rahman, M.S. State diagram of foods: Its potential use in food processing and product stability. Trends Food Sci. Technol. 2006, 17, 129–141. [Google Scholar] [CrossRef]
- Verdonck, E.; Schaap, K.; Thomas, L.C. A discussion of the principles and applications of Modulated Temperature DSC (MTDSC). Int. J. Pharm. 1999, 192, 3–20. [Google Scholar] [CrossRef]
- Forssell, P.M.; Mikkilä, J.M.; Moates, G.K.; Parker, R. Phase and glass transition behaviour of concentrated barley starch-glycerol-water mixtures, a model for thermoplastic starch. Carbohyd. Polym. 1997, 34, 275–282. [Google Scholar] [CrossRef]
- Ozmen, L.; Langrish, T. Comparison of glass transition temperature and sticky point temperature for skim milk powder. Dry. Technol. 2002, 20, 1177–1192. [Google Scholar] [CrossRef]
- Thomas, L.; Aubuchon, S. Heat Capacity Measurements Using Quasi-Isothermal MDSCTM. TA Instrum. Tech. Notes 1999, TA-230, 1–4. [Google Scholar]
- Saavedra-Leos, Z.; Leyva-Porras, C.; Araujo-Díaz, S.B.; Toxqui-Terán, A.; Borrás-Enríquez, A.J. Technological application of maltodextrins according to the degree of polymerization. Molecules 2015, 20, 21067–21081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Araujo-Díaz, S.; Leyva-Porras, C.; Aguirre-Bañuelos, P.; Álvarez-Salas, C.; Saavedra-Leos, Z. Evaluation of the physical properties and conservation of the antioxidants content, employing inulin and maltodextrin in the spray drying of blueberry juice. Carbohydr. Polym. 2017, 167, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Leyva-Porras, C.; Saavedra–Leos, M.; López-Pablos, A.; Soto-Guerrero, J.; Toxqui-Terán, A.; Fozado-Quiroz, R. Chemical, thermal and physical characterization of inulin for its technological application based on the degree of polymerization. J. Food Process Eng. 2017, 40, e12333. [Google Scholar] [CrossRef]
- Katayama, Y.; Mizutani, T.; Utsumi, W.; Shimomura, O.; Yamakata, M.; Funakoshi, K. A first-order liquid–liquid phase transition in phosphorus. Nature 2000, 403, 170. [Google Scholar] [CrossRef] [PubMed]
- Charoenrein, S. Microstructural changes and their relationship with quality and stability of frozen foods. In Food Microstructure and Its Relationship with Quality and Stability; Elsevier: Duxford, UK, 2018; pp. 123–138. [Google Scholar]
- Fukuma, Y.; Yamane, A.; Itoh, T.; Tsukamasa, Y.; Ando, M. Application of supercooling to long-term storage of fish meat. Fish. Sci. 2012, 78, 451–461. [Google Scholar] [CrossRef]
- LeBail, A.; Boillereaux, L.; Davenel, A.; Hayert, M.; Lucas, T.; Monteau, J.Y. Phase transition in foods: Effect of pressure and methods to assess or control phase transition. Innov. Food Sci. Emerg. Technol. 2003, 4, 15–24. [Google Scholar] [CrossRef]
- Kasapis, S. Building on the WLF/free volume framework: Utilization of the coupling model in the relaxation dynamics of the gelatin/cosolute system. Biomacromolecules 2006, 7, 1671–1678. [Google Scholar] [CrossRef]
- Farkas, J.; Mohácsi-Farkas, C. Application of differential scanning calorimetry in food research and food quality assurance. J. Therm. Anal. Calorim. 1996, 47, 1787–1803. [Google Scholar] [CrossRef]
- Leon, A.; Rosell, C.; De Barber, C.B. A differential scanning calorimetry study of wheat proteins. Eur. Food Res. Technol. 2003, 217, 13–16. [Google Scholar] [CrossRef]
- Sousa, I.M.N.; Mitchell, J.R.; Ledward, D.A.; Hill, S.E.; da Costa, M.L.B. Differential scanning calorimetry of lupin and soy proteins. Eur. Food Res. Technol. 1995, 201, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Del Angel, S.S.; Martínez, E.M.; Lopez, M.A.V. Study of denaturation of corn proteins during storage using differential scanning calorimetry. Food Chem. 2003, 83, 531–540. [Google Scholar]
- Zhang, H.; Takenaka, M.; Isobe, S. DSC and electrophoretic studies on soymilk protein denaturation. J. Therm. Anal. Calorim. 2004, 75, 719–726. [Google Scholar] [CrossRef]
- Rastegari, A.A.; Buzari, B.; Pavelkic, V.; Gopcevic, K.; Petkovic, M.; Bordbar, A. Thermal denaturation of pepsin at acidic media: Using DSC, MALDI-TOF MS and PAGE techniques. Thermochim. Acta 2013, 568, 165–170. [Google Scholar] [CrossRef]
- Frydenberg, R.P.; Hammershøj, M.; Andersen, U.; Greve, M.T.; Wiking, L. Protein denaturation of whey protein isolates (WPIs) induced by high intensity ultrasound during heat gelation. Food Chem. 2016, 192, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Górska, A.; Ostrowska-Ligęza, E.; Szulc, K.; Wirkowska, M. A differential scanning calorimetric study of β-lactoglobulin and vitamin D3 complexes. J. Therm. Anal. Calorim. 2012, 110, 473–477. [Google Scholar] [CrossRef]
- Escobedo, G.; Arjona-Román, J.L.; Meléndez-Pérez, R.; Suárez-Álvarez, K.; Guzmán, C.; Aguirre-García, J.; Gutiérrez-Reyes, G.; Vivas, O.; Varela-Fascinetto, G.; Rodríguez-Romero, A. Liver exhibits thermal variations according to the stage of fibrosis progression: A novel use of modulated-differential scanning calorimetry for research in hepatology. Hepatol. Res. 2013, 43, 785–794. [Google Scholar] [CrossRef]
- Morel, B.; Varela, L.; Conejero-Lara, F. The thermodynamic stability of amyloid fibrils studied by differential scanning calorimetry. J. Phys. Chem. B 2010, 114, 4010–4019. [Google Scholar] [CrossRef]
- Savadkoohi, S.; Farahnaky, A. Dynamic rheological and thermal study of the heat-induced gelation of tomato-seed proteins. J. Food Eng. 2012, 113, 479–485. [Google Scholar] [CrossRef]
- Lee, J.H.; Kannan, G.; Kouakou, B. Concentration and distribution of conjugated linoleic acids and trans-fatty acids in small ruminant milk and meat lipids. J. Food Lipids 2006, 13, 100–111. [Google Scholar] [CrossRef]
- Knopp, M.M.; Löbmann, K.; Elder, D.P.; Rades, T.; Holm, R. Recent advances and potential applications of modulated differential scanning calorimetry (mDSC) in drug development. Eur. J. Pharm. Sci. 2016, 87, 164–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Thomas, L.C.; Schmidt, S.J. Effects of heating conditions on the glass transition parameters of amorphous sucrose produced by melt-quenching. J. Agric. Food Chem. 2011, 59, 3311–3319. [Google Scholar] [CrossRef] [PubMed]
- Hinrichs, W.L.J.; Prinsen, M.G.; Frijlink, H.W. Inulin glasses for the stabilization of therapeutic proteins. Int. J. Pharm. 2001, 215, 163–174. [Google Scholar] [CrossRef]
- Vuddanda, P.R.; Montenegro-Nicolini, M.; Morales, J.O.; Velaga, S. Effect of plasticizers on the physico-mechanical properties of pullulan based pharmaceutical oral films. Eur. J. Pharm. Sci. 2017, 96, 290–298. [Google Scholar] [CrossRef]
- Afoakwa, E.O.; Paterson, A.; Fowler, M.; Vieira, J. Characterization of melting properties in dark chocolates from varying particle size distribution and composition using differential scanning calorimetry. Food Res. Int. 2008, 41, 751–757. [Google Scholar] [CrossRef]
- Bouzidi, L.; Boodhoo, M.; Humphrey, K.L.; Narine, S.S. Use of first and second derivatives to accurately determine key parameters of DSC thermographs in lipid crystallization studies. Thermochim. Acta 2005, 439, 94–102. [Google Scholar] [CrossRef]
- Humphrey, K.L.; Narine, S.S. A comparison of lipid shortening functionality as a function of molecular ensemble and shear: Crystallization and melting. Food Res. Int. 2004, 37, 11–27. [Google Scholar] [CrossRef]
- Rizzo, G.; Norton, J.E.; Norton, I.T. Emulsifier effects on fat crystallisation. Food Struct. 2014, in press. [Google Scholar] [CrossRef]
- Schubert, M.A.; Schicke, B.C.; Müller-Goymann, C.C. Thermal analysis of the crystallization and melting behavior of lipid matrices and lipid nanoparticles containing high amounts of lecithin. Int. J. Pharm. 2005, 298, 242–254. [Google Scholar] [CrossRef]
- Tolstorebrov, I.; Eikevik, T.M.; Bantle, M. A DSC determination of phase transitions and liquid fraction in fish oils and mixtures of triacylglycerides. Food Res. Int. 2014, 58, 132–140. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, M.; Bhandari, B. Recent development in 3D food printing. Crit. Rev. Food Sci. Nutr. 2017, 57, 3145–3153. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Peng, Z.; Yan, L.; Fuh, J.Y.; Hong, G.S. 3D food printing—An innovative way of mass customization in food fabrication. Int. J. Bioprinting 2015, 1, 27–38. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, W.; Huang, D.; Fuh, J.Y.; Hong, G.S. An overview of 3D printing technologies for food fabrication. Food Bioprocess Technol. 2015, 8, 1605–1615. [Google Scholar] [CrossRef]
- Mantihal, S.; Prakash, S.; Godoi, F.C.; Bhandari, B. Optimization of chocolate 3D printing by correlating thermal and flow properties with 3D structure modeling. Innov. Food Sci. Emerg. Technol. 2017, 44, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Cañadas, O.; Casals, C. Differential scanning calorimetry of protein–lipid interactions. In Lipid-Protein Interactions; Springer: New York, NY, USA, 2013; pp. 55–71. [Google Scholar]
- Stonehouse, G.; Evans, J. The use of supercooling for fresh foods: A review. J. Food Eng. 2015, 148, 74–79. [Google Scholar] [CrossRef] [Green Version]
- Jeremiah, L.; Gibson, L. The influence of storage and display conditions on the retail properties and case-life of display-ready pork loin roasts. Meat Sci. 1997, 47, 17–27. [Google Scholar] [CrossRef]
- Plumb, K. Continuous processing in the pharmaceutical industry: Changing the mind set. Chem. Eng. Res. Des. 2005, 83, 730–738. [Google Scholar] [CrossRef]
- Villafuerte Robles, L. Los excipientes y su funcionalidad en productos farmacéuticos sólidos. Rev. Mex. Cienc. Farm. 2011, 42, 18–36. [Google Scholar]
- Patel, N.K.; Patel, I.J.; Cutie, A.J.; Wadke, D.A.; Monkhouse, D.C.; Reier, G.E. The Effect of Selected Direct Compression Excipients on the Stability of Aspirin as a Model Hydrolyzable Drug. Drug Dev. Ind. Pharm. 1988, 14, 77–98. [Google Scholar] [CrossRef]
- Slade, L.; Levine, H. Non-equilibrium behavior of small carbohydrate-water systems. Pure Appl. Chem. 1988, 60, 1841–1864. [Google Scholar] [CrossRef]
- Hoppu, P. Characterisation and Processing of Amorphous Binary Mixtures with Low Glass Transition Temperature. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2008. [Google Scholar]
- Shamblin, S.L.; Tang, X.; Chang, L.; Hancock, B.C.; Pikal, M.J. Characterization of the Time Scales of Molecular Motion in Pharmaceutically Important Glasses. J. Phys. Chem. B 1999, 103, 4113–4121. [Google Scholar] [CrossRef]
- Gombás, Á.; Antal, I.; Szabó-Révész, P.; Marton, S.; Erõs, I. Quantitative determination of crystallinity of alpha-lactose monohydrate by near infrared spectroscopy (NIRS). Int. J. Pharm. 2003, 256, 25–32. [Google Scholar] [CrossRef]
- Smith, G.; Hussain, A.; Bukhari, N.I.; Ermolina, I. Quantification of residual crystallinity in ball milled commercially sourced lactose monohydrate by thermo-analytical techniques and terahertz spectroscopy. Eur. J. Pharm. Biopharm. 2015, 92, 180–191. [Google Scholar] [CrossRef] [PubMed]
- Van den Mooter, G. The use of amorphous solid dispersions: A formulation strategy to overcome poor solubility and dissolution rate. Drug Discov. Today Technol. 2012, 9, e79–e85. [Google Scholar] [CrossRef]
- DeCamp, W. Regulatory considerations in crystallisation processes for bulk pharmaceutical chemicals—A reviewer’s perspective. In Crystal Growth of Organic Materials, ACS Proceedings Series; American Chemical Society: Washington, DC, USA, 1996; pp. 66–71. [Google Scholar]
- Dunitz, J.D.; Bernstein, J. Disappearing polymorphs. Acc. Chem. Res. 1995, 28, 193–200. [Google Scholar] [CrossRef]
- Rodríguez-hornedo, N.; Murphy, D. Significance of controlling crystallization mechanisms and kinetics in pharmaceutical systems. J. Pharm. Sci. 1999, 88, 651–660. [Google Scholar] [CrossRef]
- Davey, R.; Allen, K.; Blagden, N.; Cross, W.; Lieberman, H.; Quayle, M.; Righini, S.; Seton, L.; Tiddy, G. Crystal engineering-nucleation, the key step. CrystEngComm 2002, 4, 257–264. [Google Scholar] [CrossRef]
- Rodríguez-Hornedo, N.; Murphy, D. Surfactant-facilitated crystallization of dihydrate carbamazepine during dissolution of anhydrous polymorph. J. Pharm. Sci. 2004, 93, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Zhang, G.G.; Gu, C.; Zell, M.T.; Burkhardt, R.T.; Munson, E.J.; Grant, D.J. Crystallization and transitions of sulfamerazine polymorphs. J. Pharm. Sci. 2002, 91, 1089–1100. [Google Scholar] [CrossRef]
- Haleblian, J.; McCrone, W. Pharmaceutical applications of polymorphism. J. Pharm. Sci. 1969, 58, 911–929. [Google Scholar] [CrossRef] [PubMed]
- Cui, Y. A material science perspective of pharmaceutical solids. Int. J. Pharm. 2007, 339, 3–18. [Google Scholar] [CrossRef] [PubMed]
- Jadhav, N.R.; Gaikwad, V.L.; Nair, K.J.; Kadam, H.M. Glass transition temperature: Basics and application in pharmaceutical sector. Asian J. Pharm. (AJP) 2014, 3. [Google Scholar] [CrossRef]
- Yu, L. Amorphous pharmaceutical solids: Preparation, characterization and stabilization. Adv. Drug Deliv. Rev. 2001, 48, 27–42. [Google Scholar] [CrossRef]
- Moes, J.; Koolen, S.; Huitema, A.; Schellens, J.; Beijnen, J.; Nuijen, B. Pharmaceutical development and preliminary clinical testing of an oral solid dispersion formulation of docetaxel (ModraDoc001). Int. J. Pharm. 2011, 420, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Murdande, S.B.; Pikal, M.J.; Shanker, R.M.; Bogner, R.H. Solubility advantage of amorphous pharmaceuticals: I. A thermodynamic analysis. J. Pharm. Sci. 2010, 99, 1254–1264. [Google Scholar] [CrossRef]
- Baghel, S.; Cathcart, H.; Redington, W.; O’Reilly, N.J. An investigation into the crystallization tendency/kinetics of amorphous active pharmaceutical ingredients: A case study with dipyridamole and cinnarizine. Eur. J. Pharm. Biopharm. 2016, 104, 59–71. [Google Scholar] [CrossRef]
- Gunaseelan, S.; Rao, R.; Manimaran, A.; Ramu, E.; Sivakumar, B. Determination of traces of amorphous cefuroxime axetil content in cefuroxime axetil crystalline drug substance using modulated differential scanning calorimetry (MDSC). J. Chem. Pharm. Res. 2012, 4, 4743–4751. [Google Scholar]
- Lin, Y.; Shi, Z.; Wildfong, P.L. Thermal conductivity measurements for small molecule organic solid materials using modulated differential scanning calorimetry (MDSC) and data corrections for sample porosity. J. Pharm. Biomed. Anal. 2010, 51, 979–984. [Google Scholar] [CrossRef]
- Chadha, R.; Bhandari, S. Drug–excipient compatibility screening—Role of thermoanalytical and spectroscopic techniques. J. Pharm. Biomed. Anal. 2014, 87, 82–97. [Google Scholar] [CrossRef]
- Yu, D.; Branford-White, C.; Li, L.; Wu, X.; Zhu, L. The compatibility of acyclovir with polyacrylonitrile in the electrospun drug-loaded nanofibers. J. Appl. Polym. Sci. 2010, 117, 1509–1515. [Google Scholar] [CrossRef]
- Wang, X.; Yu, D.; Li, X.; Bligh, S.A.; Williams, G.R. Electrospun medicated shellac nanofibers for colon-targeted drug delivery. Int. J. Pharm. 2015, 490, 384–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kommavarapu, P.; Maruthapillai, A.; Palanisamy, K.; Teja Koya, R. Physical characterization and dissolution performance assessment of Etravirine solid dispersions prepared by spray drying process. Pak. J. Pharm. Sci. 2016, 29, 2023–2031. [Google Scholar] [PubMed]
- Kelleher, J.; Gilvary, G.; Madi, A.; Jones, D.; Li, S.; Tian, Y.; Almajaan, A.; Senta-Loys, Z.; Andrews, G.; Healy, A. A comparative study between hot-melt extrusion and spray-drying for the manufacture of anti-hypertension compatible monolithic fixed-dose combination products. Int. J. Pharm. 2018, 545, 183–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dave, R.H.; Shah, D.A.; Patel, P.G. Development and evaluation of high loading oral dissolving film of aspirin and acetaminophen. J. Pharm. Sci. Pharmacol. 2014, 1, 112–122. [Google Scholar] [CrossRef]
- Boller, A.; Schick, C.; Wunderlich, B. Modulated differential scanning calorimetry in the glass transition region. Thermochim. Acta 1995, 266, 97–111. [Google Scholar] [CrossRef]
- Coleman, N.J.; Craig, D.Q. Modulated temperature differential scanning calorimetry: A novel approach to pharmaceutical thermal analysis. Int. J. Pharm. 1996, 135, 13–29. [Google Scholar] [CrossRef]
- Bottom, R. The role of modulated temperature differential scanning calorimetry in the characterisation of a drug molecule exhibiting polymorphic and glass forming tendencies. Int. J. Pharm. 1999, 192, 47–53. [Google Scholar] [CrossRef]








© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leyva-Porras, C.; Cruz-Alcantar, P.; Espinosa-Solís, V.; Martínez-Guerra, E.; Piñón-Balderrama, C.I.; Compean Martínez, I.; Saavedra-Leos, M.Z. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers 2020, 12, 5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010005
Leyva-Porras C, Cruz-Alcantar P, Espinosa-Solís V, Martínez-Guerra E, Piñón-Balderrama CI, Compean Martínez I, Saavedra-Leos MZ. Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries. Polymers. 2020; 12(1):5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010005
Chicago/Turabian StyleLeyva-Porras, César, Pedro Cruz-Alcantar, Vicente Espinosa-Solís, Eduardo Martínez-Guerra, Claudia I. Piñón-Balderrama, Isaac Compean Martínez, and María Z. Saavedra-Leos. 2020. "Application of Differential Scanning Calorimetry (DSC) and Modulated Differential Scanning Calorimetry (MDSC) in Food and Drug Industries" Polymers 12, no. 1: 5. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12010005