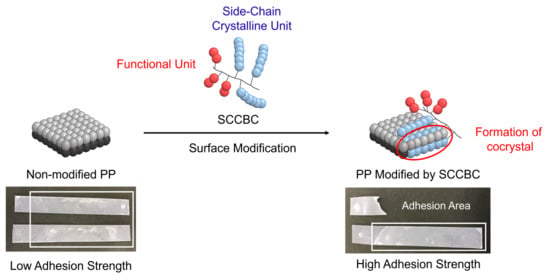
Enhancement of the Surface Properties on Polypropylene Film Using Side-Chain Crystalline Block Copolymers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Synthesis of the SCCBCs
2.3. Surface Modification of the PP Film Using SCCBCs
2.4. Characterization
3. Result and Discussion
3.1. Effect of the Surface Modification Conditions on the Adhesive Properties of PP Modified with BHA-TBAEMA
3.2. Surface Analysis of the Non-Modified PP and PP Modified with BHA-TBAEMA
3.3. Plausible Mechanism for PP Modification with SCCBC
3.4. Enhancement of the Hydrophilicity on the PP Surface by Modification with BHA-DEEA
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boone, J.; Lox, F.; Pottie, S. Deficiencies of polypropylene in its use as a food-packaging material—A review. Packag. Technol. Sci. 1993, 6, 277–281. [Google Scholar] [CrossRef]
- Chen, K.-Y.; Zhou, N.-Q.; Liu, B.; Jin, G. Improved mechanical properties and structure of polypropylene pipe prepared under vibration force field. J. Appl. Polym. Sci. 2009, 114, 3612–3620. [Google Scholar] [CrossRef]
- Chattopadhyay, R. Textile rope—A review. Indian J. Fibre Text. Res. 1997, 22, 360–368. [Google Scholar]
- Liu, G.; Qiu, G. Study on the mechanical and morphological properties of toughened polypropylene blends for automobile bumpers. Polym. Bull. 2013, 70, 849–857. [Google Scholar] [CrossRef] [Green Version]
- Stewart, R.; Goodship, V.; Guild, F.; Green, M.; Farrow, J. Investigation and demonstration of the durability of air plasma pre-treatment on polypropylene automotive bumpers. Int. J. Adhes. Adhes. 2005, 25, 93–99. [Google Scholar] [CrossRef]
- Seidi, F.; Movahedifar, E.; Naderi, G.; Akbari, V.; Ducos, F.; Shamsi, R.; Vahabi, H.; Saeb, M.R. Flame retardant polypropylenes: A review. Polymers 2020, 12, 1701. [Google Scholar] [CrossRef]
- Hu, W.; Zhang, Z.; Zhu, L.; Wen, Y.; Zhang, T.; Ren, P.; Wang, F.; Ji, Z. Combination of polypropylene mesh and in situ injectable mussel-inspired hydrogel in laparoscopic hernia repair for preventing post-surgical adhesions in the piglet model. ACS Biomater. Sci. Eng. 2020, 6, 1735–1743. [Google Scholar] [CrossRef]
- Li, J.; Huang, Y.; Zhang, S.; Jia, W.; Wang, X.; Guo, Y.; Jia, D.; Wang, L. Decoration of silica nanoparticles on polypropylene separator for lithium-sulfur batteries. ACS Appl. Mater. Interfaces 2017, 9, 7499–7504. [Google Scholar] [CrossRef]
- Haigh, J.N.; Dargaville, T.R.; Dalton, P.D. Additive manufacturing with polypropylene microfibers. Mater. Sci. Eng. C 2017, 77, 883–887. [Google Scholar] [CrossRef] [Green Version]
- Lin, Y.; Li, H.; Wang, Q.; Gong, Z.; Tao, J. Effect of plasma surface treatment of aluminum alloy sheet on the properties of Al/Gf/PP laminates. Appl. Surf. Sci. 2020, 507, 145062. [Google Scholar] [CrossRef]
- Cortés, P.; Cantwell, W.J. The fracture properties of a fibre-metal laminate based on magnesium alloy. Compos. B Eng. 2006, 37, 163–170. [Google Scholar] [CrossRef]
- Kim, K.J.; Kim, D.; Choi, S.H.; Chung, K.; Shin, K.S.; Barlat, F.; Oh, K.H.; Youn, J.R. Formability of AA5182/polypropylene/AA5182 sandwich sheets. J. Mater. Process. Technol. 2003, 139, 1–7. [Google Scholar] [CrossRef]
- Chaffin, K.A.; Knutsen, J.S.; Brant, P.; Bates, F.S. High-strength welds in metallocene polypropylene/polyethylene laminates. Science 2000, 288, 2187–2190. [Google Scholar] [CrossRef]
- Maimaitiming, A.; Zhang, M.; Tan, H.; Wang, M.; Zhang, M.; Hu, J.; Xing, Z.; Wu, G. High-strength triple shape memory elastomers from radiation-vulcanized polyolefin elastomer/polypropylene blends. ACS Appl. Polym. Mater. 2019, 1, 1735–1748. [Google Scholar] [CrossRef]
- Liao, J.; Brosse, N.; Pizzi, A.; Hoppe, S.; Xi, X.; Zhou, X. Polypropylene blend with polyphenols through dynamic vulcanization: Mechanical, rheological, crystalline, thermal, and UV protective property. Polymers 2019, 11, 1108. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.S.; Janke, A.; Gohs, U.; Heinrich, G. Electron-induced reactive processing of polyamide 6/polypropylene blends: Morphology and properties. Eur. Polym. J. 2018, 98, 295–301. [Google Scholar] [CrossRef]
- Etcheverry, M.; Barbosa, S.E. Glass fiber reinforced polypropylene mechanical properties enhancement by adhesion improvement. Materials 2012, 5, 1084–1113. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.-X.; Yu, Z.-Z.; Xie, X.-L.; Mai, Y.-W. Crystallization and impact energy of polypropylene/CaCO3 nanocomposites with nonionic modifier. Polymer 2004, 45, 5985–5994. [Google Scholar] [CrossRef]
- Manias, E.; Touny, A.; Wu, L.; Strawhecker, K.; Lu, B.; Chung, T.C. Polypropylene/montmorillonite nanocomposites. Review of the synthetic routes and materials properties. Chem. Mater. 2001, 13, 3516–3523. [Google Scholar] [CrossRef] [Green Version]
- Chung, T.C.M. Expanding polyethylene and polypropylene applications to high-energy areas by applying polyolefin-bonded antioxidants. Macromolecules 2019, 52, 5618–5637. [Google Scholar] [CrossRef]
- Chen, H.; Kong, L.; Wang, Y. Enhancing the hydrophilicity and water permeability of polypropylene membranes by nitric acid activation and metal oxide deposition. J. Membr. Sci. 2015, 487, 109–116. [Google Scholar] [CrossRef]
- Gu, H.; Wu, J.; Chan, P.; Turcotte, G.; Ye, T. Hydrophilicity modification of polypropylene microfiltration membrane by ozonation. Chem. Eng. Res. Des. 2012, 90, 229–237. [Google Scholar] [CrossRef]
- Mandolfino, C. Polypropylene surface modification by low pressure plasma to increase adhesive bonding: Effect of process parameters. Surf. Coat. Technol. 2019, 366, 331–337. [Google Scholar] [CrossRef]
- Sato, T.; Akiyama, H.; Horiuchi, S.; Miyamae, T. Characterization of the polypropylene surface after atmospheric pressure N2 plasma irradiation. Surface Sci. 2018, 677, 93–98. [Google Scholar] [CrossRef]
- Gourianova, S.; Willenbacher, N.; Kutschera, M. Chemical force microscopy study of adhesive properties of polypropylene films: Influence of surface polarity and medium. Langmuir 2005, 21, 5429–5438. [Google Scholar] [CrossRef]
- Süzer, S.; Argun, A.; Vatansever, O.; Aral, O. XPS and water contact angle measurements on aged and corona-treated PP. J. Appl. Polym. Sci. 1999, 74, 1846–1850. [Google Scholar] [CrossRef]
- Strobel, M.; Branch, M.C.; Ulsh, M.; Kapaun, R.S.; Kirk, S.; Lyons, C.S. Flame surface modification of polypropylene film. J. Adhes. Sci. Technol. 1996, 10, 513–539. [Google Scholar] [CrossRef]
- Sutherland, I.; Brewis, D.M.; Health, R.J.; Sheng, E. Modification of polypropylene surfaces by flame treatment. Surf. Interface Anal. 1991, 17, 507–510. [Google Scholar] [CrossRef]
- Dai, J.; Liang, M.; Ren, P.; Fu, Y.; Wang, F.; Ge, X.; Zhang, T. Surface modification of polypropylene with porous polyacrylamide/polydopamine composite coating. Mater. Lett. 2020, 266, 127487. [Google Scholar] [CrossRef]
- Li, X.; Zhou, Q.; Yang, H. Ultrafast and stable adsorption-desorption performance for recovery of valuable rare-earth ions using high-density polyacrylic acid brush-grafted polypropylene fibers optimized by RSM models. Ind. Eng. Chem. Res. 2020, 59, 7746–7754. [Google Scholar] [CrossRef]
- Ohkubo, K.; Asahara, H.; Inoue, T. Photochemical C-H oxygenation of side-chain methyl groups in polypropylene with chlorine dioxide. Chem. Commun. 2019, 55, 4723–4726. [Google Scholar] [CrossRef] [PubMed]
- Hiromori, T.; Hirai, S.; Phanthong, P.; Nakano, R.; Sekiguchi, H.; Yao, S. Dependence of viscosity and transition temperatures of a thermal rheological fluid system on the molecular weight of the side-chain crystalline block copolymer dispersant. Mater. Chem. Phys. 2020, 243, 122605. [Google Scholar] [CrossRef]
- Hirai, S.; Ishimoto, S.; Phanthong, P.; Yao, S. Development of surface properties of ultra-high-molecular-weight polyethylene film using side-chain crystalline block copolymers. J. Polym. Eng. 2020, 40, 231–236. [Google Scholar] [CrossRef]
- Hirai, S.; Ishimoto, S.; Obuchi, H.; Yao, S. Improving the adhesion of polyethylene surfaces using side-chain crystalline block copolymer. J. Adhes. Sci. Technol. 2019, 33, 2567–2578. [Google Scholar] [CrossRef]
- Miho, Y.; Hirai, S.; Nakano, R.; Sekiguchi, H.; Yao, S. Modification of polyethylene using side-chain crystalline block copolymer and evaluation of hydrophilicity. Polym. J. 2018, 50, 439–445. [Google Scholar] [CrossRef]
- Devasahayam, S.; Sahajwalla, V.; Sng, M. Investigation into failure in mining wire ropes—Effect of crystallinity. Open J. Org. Polym. Mater. 2013, 3, 34–40. [Google Scholar] [CrossRef] [Green Version]










Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hirai, S.; Phanthong, P.; Okubo, H.; Yao, S. Enhancement of the Surface Properties on Polypropylene Film Using Side-Chain Crystalline Block Copolymers. Polymers 2020, 12, 2736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112736
Hirai S, Phanthong P, Okubo H, Yao S. Enhancement of the Surface Properties on Polypropylene Film Using Side-Chain Crystalline Block Copolymers. Polymers. 2020; 12(11):2736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112736
Chicago/Turabian StyleHirai, Sho, Patchiya Phanthong, Hikaru Okubo, and Shigeru Yao. 2020. "Enhancement of the Surface Properties on Polypropylene Film Using Side-Chain Crystalline Block Copolymers" Polymers 12, no. 11: 2736. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112736