3.3. Evaporation Rate of Free Water from CUPs
The evaporation of both water and CUP solutions are highly dependent upon surface area. If the sample does not wet the platinum pan it can result in changing areas caused by the sample size and contact angle. To evaluate this, two platinum flags were cleaned, and one had 15 microliters of deionized water placed on its surface and the other had a 10% Polymer 4 CUP solution at the same volume placed on it.
Figure 3 shows the image of the flags at time zero just after application and the third image after the CUP solution was dry. It can be seen that water wet the platinum partially, but the CUP solution wet much better. Once dry the CUP sample formed a relatively even coating which cracked due to poor adhesion and low crystal lattice energy. This experiment indicates that the evaporation should be representative, even to the end for CUP since it evaporates evenly.
In order to investigate the CUP particles’ effect on the evaporation rate, a 5.47% Polymer 1 solution was measured following the same protocol as with water, and compared with deionized water, as shown in
Figure 4.
The Polymer 1 solution evaporated faster than deionized water in the beginning, and kept decreasing along the isothermal process, with multiple changes of evaporation rate reduction being observed. These complexities indicated that there was more than one factor involved during the isothermal process. The study separated the evaporation process into five segments designated as I, II, III, IV, and V, shown in
Figure 5.
Segment I was the initial time frame of 480 s, before major compositional changes occurred. Segment II is for the range from Segment I until Manning condensation occurs. Segment III covers Manning condensation. Segment IV is the gelation of the solution and Segment V is the loss of the last water including surface water.
The evaporation rate of CUP solutions was not constant. Therefore, in order to investigate the CUP’s effect on the evaporation rate in the beginning, the evaporation rate of various polymer solutions with multiple molarities were determined immediately after the pan settled down, at 480 s (Segment I), as shown in
Figure 6.
Polymer 1, 2 and 3 have the same monomer ratio but different surface charge density and molecular weight. It was observed that, with the same molarity, polymers with higher molecular weight and surface charge density have higher evaporation rate. If comparing Polymer 2, 4 and 5, which have the same surface charge density, the polymer with larger molecular weight had a faster evaporation rate. Van De Mark et al. found that with the presence of CUP particles, the surface tension was lower than deionized water, and it was proportional to the number of charges on the particle surface [
28]. The surface tension reduction was similar but smaller than that observed for typical surfactants [
29]. This surface tension reduction was also observed for polyelectrolyte systems [
30]. Polymer 3 evaporated faster than Polymer 2 and 1, due to more charges, which is the same for Polymers 2, 4, and 5. It was also observed that for each CUP solution, the evaporation rate was higher for the solutions with a higher initial molarity because the higher initial molarity having more charges. However, the effect of surface tension should not be a major factor for evaporation, but it will have an effect on interfacial mobility. In order to further investigate the surface effect, the relation between surface tension and the evaporation rate of aqueous salts was examined, as shown in
Table 2.
Sodium chloride was chosen since it causes an increase in the surface tension and sodium acetate, which has a carboxylate like CUP, causes a decrease in surface tension. The evaporation rate difference from water divided by the surface tension difference from water was used to evaluate the effect each had on the two, ΔR/Δγ. It was shown that, with less surface tension, sodium acetate solution evaporated slower than deionized water, due to the salts [
31,
32]. Also, the evaporation rate change was moderately lower than the change in surface tension for all four values. This data indicates that the primary effect on increasing the evaporation rate of CUPs is not surface tension.
Table 3 gives the surface tension and evaporation rate for CUP solutions of Polymer 1–6 at 2 mM. It should be noted that the change in the surface tension for these polymers are about 500 times higher than that for sodium acetate at the same concentration. Therefore, the molar concentration of CUP may not be a simple relationship.
Table 3 also gives the number of carboxylate groups on each CUP. The number of carboxylates were partially responsible for the larger effect of both evaporation rate and surface tension. The chains of the CUP particle are not free to move and thus their relationship to each other define the “more hydrophobic” regions from the carboxylate. These hydrophobic regions are larger than those of the methyl group of the acetate ion. However, the more hydrophobic surface is dominated by the ester groups and likely some of the methyl groups of the backbone and ester. The surface tension of surfactant carboxylates becomes more effective as the aliphatic chain increases.
The use of percent solids as well as molarity and weight fraction,
XCUP are relevant to different aspects of this study Equation (2) relates these terms.
where
Mw is the molecular weight of the polymer,
ρs is the density of CUP solution,
c is the molarity, and
XCUP is the weight fraction of CUP solids.
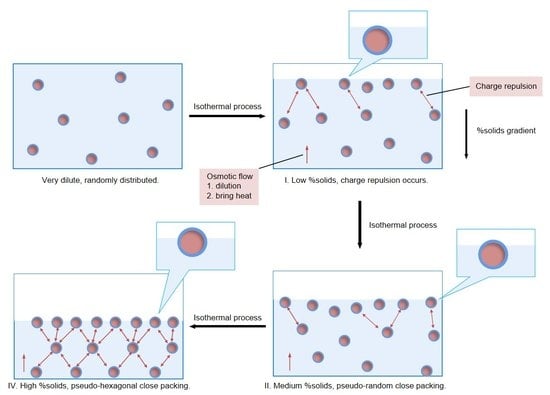
When a very dilute CUP solution was at its equilibrium condition, the solution was homogeneous and CUP particles were randomly distributed and stabilized by the combination of Brownian motion, solvation by water and charge repulsion between particles. Assuming that each CUP occupies an average cubic volume in solution, which gives the largest distance between particles. At a given percent solids, the distance between two CUP particles was estimated by Equation (3).
where
r is the distance between two particles,
M is the molecular weight of CUP,
XCUP is the weight fraction of CUP,
ρ is the density of solution,
d is the size of the CUP particle,
NA is Avogadro constant.
The distance between two CUP particles was determined to be from 5.5 to 8.8 nm depending on the particle size at 5% solids. The electrostatic effective distance between two CUP particles can be estimated by Equations (4)–(6) [
33,
34,
35].
where
I is the ionic strength,
M is the molarity,
nc is the number of carboxylate groups per CUP,
ɛ0 is the permittivity of free space,
ɛr is the dielectric constant for water,
kB is the Boltzmann constant,
e is the elementary charge, and
κ−1 is Debye length.
The assumption is that we have a single point charge separated by water. As
Figure 7 shows, the effective distance was always larger than the estimated inter-particle distance, which indicated that the electrostatic repulsion force occurred at a CUP concentration of 1% and higher. At a constant percent solids, the CUP with smaller particle size tends to have a larger difference between the effective distance and inter-particle distance, due to a higher number of particles that results in a higher repulsion force. Polymer 1 has a higher effective distance to inter-particle distance ratio than Polymer 3, because of a larger number of particles at the same percent solids.
Due to coulomb’s law, the repulsion force is proportional to
1/r2 [
36], where r is the distance between two charges. Consider each CUP particle as a point charge, and assume an r value of 9 nm, the electrostatic repulsion force for Polymer 1 is 2.85 × 10
−12 N, while the surface tension of water is 7.22 × 10
−11 N/nm and surface tension for 5.47% Polymer 1 solution is 7.08 × 10
−11 N/nm. Since each CUP particle has multiple charges (29.3 to 124.1 charges per particle for Polymers 1 and 3 respectively), the actual repulsion force was expected to be much larger than 2.85 × 10
−12 N. Therefore, at 5% solids, the charge repulsion between CUP particles should be strong enough to cause deformation of the air-water interface as shown in
Scheme 1.
The three models indicate A: CUP with surface water and a layer of air/surface water, B: CUP with a layer of surface water, and C: Cup particle with no water. Model C can be eliminated, because CUP particles are highly hydrophilic on the surface, and have a layer of strongly associated surface water [
27]. If model C were the case, all the evaporation rate would be due to edge effects on the surface tension and the loss of surface area occupied by the CUP particles would reduce the evaporation rate. Therefore, with the presence of CUP particles, the interface water deformed causing a decrease in surface tension, according to the Gibbs isotherm, and an increase in surface area. Assuming all the observed increase in the evaporation rate were contributed by increased surface area at the interface, the increased ratio of evaporation rate should be proportional to the increased surface area. At a given percent solids, the degree of interface water deformation (
Scheme 2) could be calculated by Equation (7).
where
h is the height of the interface water deformation, Δ
R is the increased evaporation rate compared with water,
R is the evaporation rate of the CUP solution,
Mw is the molecular weight of CUP,
ρ is the density of the solution,
XCUP is the weight fraction of CUP.
The h was calculated to be from 0.70 to 1.27 nm with the range of about 5 to 20% solids, h depends on the percent solids and molecular weight of CUP particles. The particle size of CUPs ranges from 4.02 to 6.83 nm, due to the moderate repulsive force, interfacial water deformation may be the major contributor. The surface tension on the deformational region may be lower than normal water and the circumference region will have a significantly lower surface tension. It is most likely that Model A or B is the correct one and that contribution from the increased surface area is the cause of the enhanced evaporation at low concentrations. It is well known that polymers in solution reduce the vapor pressure of the solvent. This would in general reduce the evaporation rate of a CUP solution. The increased area must overcome this small reduction in vapor pressure also.
In Segment I, the main factor that dominated the evaporation rate was the increased surface area which increased the rate. At a given molarity, the CUP with larger particle size has shorter inter-particle distance, resulting in a higher charge repulsion force, that increases the amount of interfacial water deformation, h. In addition, more charges on the CUP surface caused more surface tension reduction, the combination of these two effects showed a higher evaporation rate. Looking back to
Figure 4, 25.4k, Polymer 4 has a similar particle size as 28.9k, Polymer 1 and the CUP surface tension was lower for Polymer 4 and the interfacial deformation was greater due to the larger charge repulsion, therefore it showed a higher evaporation rate. Polymer 6, 49.7k has the lowest charge density but a higher molecular weight, particle size, than Polymer 1. The surface tensions for Polymer 1 and 6 are similar and the evaporation rate for Polymer 6 is slightly higher. This is because with the same molarity, Polymer 6 has a shorter inter-particle distance due to the larger particle size, that resulted in a higher charge repulsion. The interfacial water deformation for Polymer 6 would be expected to be greater thus showing a higher evaporation rate.
As water continuously evaporated during the isothermal process, the temperature at the interface decreased due to the lost heat of vaporization and the surface molarity/percent solids of CUP particles at the interface became higher than the bulk solution, Segment II. As the surface molarity/percent solids increases, it sets up an osmotic gradient with the bulk solution. The osmotic gradient draws water to the surface to dilute the CUPs [
37,
38]. The movement of water to the surface not only dilutes the CUP at the air interface but also brings heat to reestablish equilibrium. At the same time, CUP particles experience a higher charge repulsion and move toward the bottom through translational diffusion at low concentration. The reduced temperature at the interface decreases the evaporation rate, and the increased molarity provided higher charge repulsion to create an increase in the interfacial water deformation that will increase the water evaporation rate. However, the evaporation rate largely depends on the diffusion rate of water molecules to the interface [
11]. The viscosity in the interfacial region will be increasing with the increasing of CUP molarity/percent solids, due to the secondary electroviscous effect [
39], which was demonstrated by Van De Mark et al. [
40]. The increased viscosity slowed movements of both the water and CUP particles, which explains a slower observed evaporation rate.
The diffusion coefficient could be determined by Stokes–Einstein equation [
41].
where
KB is Boltzmann constant,
T is the absolute temperature,
η is the viscosity of the solution, and
r is the radius of CUP particle.
Table 4 gives the diffusion coefficient for the six polymers at 5% and 10% at 298.15 K. The lower diffusion coefficient at higher concentrations is due to charge repulsion increasing the viscosity and drops faster as the concentration increases approaching infinity at the gel point.
The evaporation rate of Polymer 4 solution with initial percent solids of 4.71%, 10.34%, 16.92% and 20.16% in the first 2500 s were evaluated as an example, shown in
Figure 8.
It was shown that, the higher initial percent solids of CUP solution, the faster the initial evaporation rate was, due to the increased surface area, Segment I. However, when the water started to evaporate, the evaporation rate of all CUP solutions decreased, except the 4.71% which retained its evaporation rate for a longer time before decreasing. The change was more obvious for higher initial percent solids CUP solutions. Because the higher percent solids solution has higher viscosity, that resulted in a slower movement of particles and water molecules in Segment II. The 4.71% solution was dilute enough that the translational diffusion and osmotic flow kept the surface CUP concentration lower for a longer time as the evaporation progressed. The low mobility of particles and water molecules further enhanced the CUP particles stacking at interface, increasing the viscosity, and reducing the evaporation rate. This observation was further investigated by comparing the evaporation rate when two different initial percent solids solution were evaporated to the same percent solids. The evaporation rate of Polymer 1 and Polymer 4 solutions with different initial percent solids were evaluated during the drying process at the same solids content, as shown in
Figure 9.
It was seen that when concentrated to the same percent solids, the evaporation rate of the low initial percent solids solution was lower. The low solids sample must first loose significant water which creates a higher concentration of CUP particles at the surface which increases the viscosity lessening the diffusion of water to the surface and slowing the movement of CUP particles away from the surface as well as lowering the surface temperature. For the lower concentration the total solution thickness is decreasing with time as does the higher concentration, however the rate of change in the thickness is almost twice as much for the lower concentration. The shorter distance will also influence the result by reducing the osmotic flow since the liquid thickness increases the opportunity to set up osmotic gradients. Polymer 1 shows a greater difference in evaporation rate than Polymer 4. The rate differences may be due to the higher charge density of Polymer 4 forcing the particles to rearrange positions more rapidly and increasing viscosity. Polymer 4 has a slightly lower mass which makes the charge effect even more meaningful.
As evaporation progressed in Segment II, the movement of water molecules and CUP particles caused by osmotic pressure and charge repulsion were the dominant driving forces in this segment. As the concentration increased the viscosity reduced the osmotic flow which in turn allowed the temperature of the surface to fall lower since the rate of warm water being transferred to the surface slowed.
The ionic force between particles forces CUP particles down from the surface by each one pushing down the particle below it, which helped to minimize osmotic differences. The ionic force also increases the vertical displacement of the CUP particles at the air interface as the CUP concentration increases. The air surface area with free water decreases and the area of CUP surface water increases and dominates evaporation as the solids content rises. When the concentration at or near the surface hits about 20% solids the CUP surface ions begin to undergo Manning type condensation which lowers the effective charge. When this condensation begins to occur the CUP particles can increase their packing concentration and reduce the repulsion on their neighbor as well as limit the CUP penetration through the air interface.
In addition, particle size is another important factor, because it directly influences the diffusion rate of CUP particles. The Polymer 2 and Polymer 4 solutions with similar molarity were compared, as shown in
Figure 10.
It was seen that, in Segment I, the Polymer 2 solution evaporated slightly faster due to more charges per particle than Polymer 4. However, Polymer 2 solution started to show a larger and larger evaporation rate reduction as compared to Polymer 4 solution through Segment II. This reduction was due to a larger particle size diffusing slower, which caused more particles to stack up at the air-water interface. This also increased the viscosity at the interface, and further decreased the diffusion rate of particles, and therefore, Polymer 2 presented a slower evaporation rate through Segment II.
Previous studies have demonstrated that when the percent solids increased above 20%, inter-molecular counterion condensation occurred, segment III [
27]. Increasing the CUP percent solids also increased the counterion concentration, which condensed on the CUP surface reducing its effective charge [
29]. The phenomenon known as Manning condensation (counterion condensation) is widely accepted in charge stabilized colloidal suspensions [
42]. The inter-molecular counterion condensation causes the effective charge to be lower than the bare surface charge and allows more CUP particles with better packing at the air-water interface. At the same time, the total number of charged groups at the air-water interface increases because only a small fraction of the charged groups on the CUP surface undergo Manning condensation. The inter-molecular counterion condensation decreases the charge repulsion effect to a degree, therefore, decreases the mobility of CUP particles to the bottom as a result of charge repulsion.
Using Polymer 6 as an example, it was shown that with the increasing of the initial percent solids, the inter-molecular counterion condensation occurs earlier in the time frame. This drop in the rate can be observed in
Figure 11. The darker line drops to an evaporation rate of 4 micrograms per second first while the 4.35% occurs much later. Segment III begins with inter-molecular counterion condensation and ends with gelation as random close packing, RCP, which is marked with a red line in
Figure 10. Starting at low concentration it requires more time to reach RCP as well as the start of Manning condensation. Once gelled, the CUP particles cannot move translationally, but they can move as a unity, shrinking all the spaces between particles uniformly to avoid significant stress development. Rapid evaporation has been noted to cause crack development in a drying sample with the surface shrinking due to drying before the system can reestablish equilibrium.
The evaporation of water, as it approaches the end of Segment III, slowed as the percent solids of the bulk solution increased, this reduced the distance between particles in the bulk portion. The particles reached a pseudo random close packing state which was defined as the gel point of CUP, and then with a small additional loss of water became pseudo hexagonal close packing (HCP), Segment IV. The term pseudo HCP was used since the particles have a distribution of diameters and charges so it will not be a perfect HCP lattice. As the particles formed an organized structure where each particle occupied a lattice position even in the bulk solution, the mobility of water molecules and CUP particles were highly limited [
40]. Thus, the evaporation rate decreased even faster. All water diffusion was either through the CUP surface water or through the voids between the spheres occupied by free water with the state of surface deformation having little meaning since the surface is now occupied by CUP particles with their surface water only.
3.4. Evaporation of CUP Surface Water
As water molecules continuously escaped from the interface, the particles approach each other, and the increased electrostatic repulsion tends to arrange them in positions with equal distance from the nearby particles. There are two types of packing models for spheroidal materials when the percent solids are very high, random close packing and hexagonal close packing. CUP particles will first approach random close packing as the concentration increases and slowly, through movements driven by the repulsive forces between particles approach hexagonal close packing. At this point the particles are only surrounded by surface water with a small amount of free water occupying the space in the voids, Segment IV. Many previous studies have shown that surface water has a much lower mobility, higher density [
43], and tighter association with the hydrophilic groups [
44]. Therefore, the evaporation rate of surface water is expected to show very different behavior from free water. The viscosity of the solution is close to infinity at this point and free water has to move primarily through the surface water layer. The rate of water diffusion is therefore very small and significantly reduces the rate of evaporation. There are potentially three possibilities for each packing model, shown in
Scheme 3. CUP with surface water and free water, CUP with free water and CUP only with surface water. Previous studies have eliminated models III and VI since the existence of a surface water layer has been demonstrated [
45].
In order to evaluate the evaporation process of the surface water and the last trace of free water in the voids, the percent solids of CUP particles for each packing model can be calculated by knowing the max volume fraction of random close packing, which is 0.634, and 0.7405 for hexagonal close packing [
46,
47,
48,
49,
50], according to Equations (9)–(11).
where
ϕ is the CUP volume fraction,
ρs is the density of CUP solution,
ρp is the density of CUP particle,
ϕR-max is the maximum volume fraction for random close packing,
ϕH-max is the maximum volume fraction for hexagonal close packing,
λ is the thickness of surface water, and
r is radius of the CUP particle.
The percent solids of CUP for the possibilities of each were calculated and are shown in
Table 5.
The amount and thickness of CUP surface water has been determined by DSC [
27]. As it was discussed in
Figure 1, for the last water loss, a large reduction in the evaporation rates were observed near the end. One possible reason for this issue was an insufficient amount of solution to cover the pan bottom. In this case, 5.04% Polymer 2 and 4.35% Polymer 6 solutions were used as examples. Knowing the diameter of the pan being 9.4 mm, it was calculated that even when there is only CUP solids existing in the pan, the bottom of the pan is still fully covered with a 0.02 mm depth. By applying the results from
Table 5 to the evaporation curve,
Scheme 1 indicates that CUP solutions dry relatively uniform wetting the platinum pan. The CUP solution surface tension decreases with increasing concentration making it more wetting of the pan. Therefore, in Segment V, the evaporation rate reduction was not because of the lack of sufficient solution to cover the pan.
Figure 12 shows both the evaporation rate of Polymer 2 and 6 solution and their corresponding percent solids.
By comparing the percent solids of CUP at each slope change of the evaporation rate curve with the results in the
Table 2, we illustrate the process of how surface water and free water in the voids evaporate. The results showed that evaporation rate sharply decreased when the percent solids passes the HCP, due to the highly limited mobility of particles and water molecules. Another big evaporation rate reduction occurred at about 53%, where there is no free water between the CUP particles and two layers of surface water around CUP particles, that implied surface water doesn’t evaporate until all free water is released. The next step was at about 72% solid, where there was only one layer of surface water, due to the inner layer being more tightly hydrogen bounded to the CUP surface. Furthermore, at about 96% solid, there was another evaporation rate change.
The results imply that free water completely evaporated before surface water started to evaporate, and water molecule associated to carboxylate group are released in the end. This was demonstrated for CUP solutions with different molecular weight and surface charge density, and indicated it is molecular weight and surface charge density independent.
Sodium acetate hydrate has three waters of hydration which are held relatively strongly. Based on
Table 4, Polymer 2 and Polymer 4 should have 94.92% and 96.55% solids respectively if it had 3 waters of hydration also. As can be seen in
Figure 11 the two polymers are very close to these values. Therefore, it is highly likely that the last three waters to leave are those associated with the surface carboxylates.