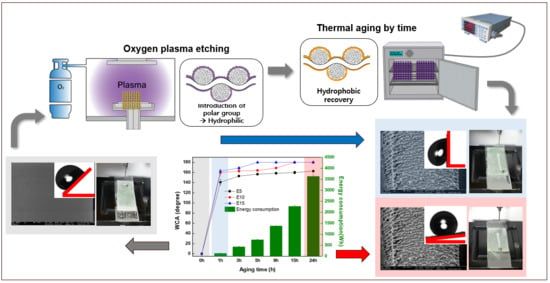
Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Fabrications
2.3. Characterization
2.3.1. Surface and Chemical Composition Analysis
2.3.2. Wettability Measurement and Self-Cleaning Test
2.3.3. Measurement of the Energy Consumption
2.3.4. Breathability and Durability Test
3. Results
3.1. Change in Surface Morphology
3.2. Changes in the Chemical Compositions
3.3. Surface Wettability at Various Thermal Aging Temperatures
3.4. Surface Wettability and Energy Consumption at Various Oxygen Plasma Etching and Thermal Aging Time
3.5. Comparison of the Shedding Angle, Sliding Angle, and Self-Cleaning Property
3.6. Surface Wettability at Various Weave Density
3.7. Breathability and Durability Test
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Feng, L.; Li, S.; Li, Y.; Li, H.; Zhang, L.; Zhai, J.; Song, Y.; Liu, B.; Jiang, L.; Zhu, D. Super-hydrophobic surfaces: From natural to artificial. Adv. Mater. 2002, 14, 1857–1860. [Google Scholar] [CrossRef]
- Jafari, N.E.; Ebrahimi, N.G.; Ayazi, M. An anti-bacterial approach to nanoscale roughening of biomimetic rice-like pattern PP by thermal annealing. Appl. Surf. Sci. 2017, 423, 1054–1061. [Google Scholar] [CrossRef]
- Bittoun, E.; Marmur, A. The role of multiscale roughness in the lotus effect: Is it essential for super-hydrophobicity? Langmuir 2012, 28, 13933–13942. [Google Scholar] [CrossRef] [PubMed]
- Jung, Y.C.; Bhushan, B. Contact angle, adhesion and friction properties of micro-and nanopatterned polymers for superhydrophobicity. Nanotechnology 2006, 17, 4970–4980. [Google Scholar] [CrossRef]
- Dalawai, S.P.; Saad Aly, M.A.; Latthe, S.S.; Xing, R.; Sutar, R.S.; Nagappan, S.; Ha, C.-S.; Kumar, S.K.; Liu, S. Recent advances in durability of superhydrophobic self-cleaning technology: A critical review. Prog. Org. Coat. 2020, 138, 105381. [Google Scholar] [CrossRef]
- Bahners, T.; Textor, T.; Opwis, K.; Schollmeyer, E. Recent approaches to highly hydrophobic textile surfaces. J. Adhes. Sci. Technol. 2008, 22, 285–309. [Google Scholar] [CrossRef]
- Shim, M.H.; Kim, J.; Park, C.H. The effects of surface energy and roughness on the hydrophobicity of woven fabrics. Text. Res. J. 2014, 84, 1268–1278. [Google Scholar] [CrossRef]
- Oh, J.-H.; Ko, T.-J.; Moon, M.-W.; Park, C.H. Nanostructured superhydrophobic silk fabric fabricated using the ion beam method. RSC Adv. 2014, 4, 38966–38973. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.; Park, C.H. Contribution of surface energy and roughness to the wettability of polyamide 6 and polypropylene film in the plasma-induced process. Text. Res. J. 2016, 86, 461–471. [Google Scholar] [CrossRef]
- Oh, J.; Park, C.H. Robust fluorine-free superhydrophobic PET fabric using alkaline hydrolysis and thermal hydrophobic aging process. Macromol. Mater. Eng. 2018, 303, 1700673. [Google Scholar] [CrossRef]
- Oh, J.-H.; Ko, T.-J.; Moon, M.-W.; Park, C.H. Nanostructured fabric with robust superhydrophobicity induced by a thermal hydrophobic ageing process. RSC Adv. 2017, 7, 25597–25604. [Google Scholar] [CrossRef] [Green Version]
- Li, J.J.; Muralikrishnan, S.; Ng, C.-T.; Yung, L.-Y.L.; Bay, B.-H. Nanoparticle-induced pulmonary toxicity. Exp. Biol. Med. 2010, 235, 1025–1033. [Google Scholar] [CrossRef] [PubMed]
- Nemmar, A.; Holme, J.A.; Rosas, I.; Schwarze, P.E.; Alfaro-Moreno, E. Recent advances in particulate matter and nanoparticle toxicology: A review of the in vivo and in vitro studies. Biomed. Res. Int. 2013, 2013, 1–22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Privett, B.J.; Youn, J.; Hong, S.A.; Lee, J.; Han, J.; Shin, J.H.; Schoenfisch, M.H. Antibacterial fluorinated silica colloid superhydrophobic surfaces. Langmuir 2011, 27, 9597–9601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Joensen, U.N.; Bossi, R.; Leffers, H.; Jensen, A.A.; Skakkebæk, N.E.; Jørgensen, N. Do perfluoroalkyl compounds impair human semen quality? Environ. Health Perspect. 2009, 117, 923–927. [Google Scholar] [CrossRef]
- Malynych, S.; Luzinov, I.; Chumanov, G. Poly(Vinyl Pyridine) as a universal surface modifier for immobilization of nanoparticles. J. Phys. Chem. B 2002, 106, 1280–1285. [Google Scholar] [CrossRef]
- Liu, Y.; Xin, J.H.; Choi, C.-H. Cotton fabrics with single-faced superhydrophobicity. Langmuir 2012, 28, 17426–17434. [Google Scholar] [CrossRef]
- Oh, J.; Park, C.H. Colorful fluorine-free superhydrophobic polyester fabric prepared via disperse dyeing process. Adv. Mater. Interfaces 2020, 7, 2000127. [Google Scholar] [CrossRef]
- Mirabedini, S.M.; Rahimi, H.; Hamedifar, S.; Mohsen Mohseni, S. Microwave irradiation of polypropylene surface: A study on wettability and adhesion. Int. J. Adhes. Adhes. 2004, 24, 163–170. [Google Scholar] [CrossRef]
- Pan, Q.; Shim, E.; Pourdeyhimi, B.; Gao, W. Highly conductive polypropylene–graphene nonwoven composite via interface engineering. Langmuir 2017, 33, 7452–7458. [Google Scholar] [CrossRef]
- Grundke, K.; Augsburg, A. On the determination of the surface energetics of porous polymer materials. J. Adhes. Sci. Technol. 2000, 14, 765–775. [Google Scholar] [CrossRef]
- Cowie, J.M.G. Glass transition temperatures of stereoblock, isotactic and atactic polypropylenes of various chain lengths. Eur. Polym. J. 1973, 9, 1041–1049. [Google Scholar] [CrossRef]
- Bhushan, B.; Her, E.K. Fabrication of superhydrophobic surfaces with high and low adhesion inspired from rose petal. Langmuir 2010, 26, 8207–8217. [Google Scholar] [CrossRef] [PubMed]
- Mak, C.M.; Yuen, C.W.M.; Ku, S.K.A.; Kan, C.W. Low-temperature plasma treatment of Tencel. J. Text. Inst. 2006, 97, 533–540. [Google Scholar] [CrossRef]
- Zimmermann, J.; Seeger, S.; Reifler, F.A. Water shedding angle: A new technique to evaluate the water-repellent properties of superhydrophobic surfaces. Text. Res. J. 2009, 79, 1565–1570. [Google Scholar] [CrossRef]
- Chu, Z.; Seeger, S. Superamphiphobic surfaces. Chem. Soc. Rev. 2014, 43, 2784–2798. [Google Scholar] [CrossRef]
- Wei, Q.; Schlaich, C.; Prévost, S.; Schulz, A.; Böttcher, C.; Gradzielski, M.; Qi, Z.; Haag, R.; Schalley, C.A. Supramolecular polymers as surface coatings: Rapid fabrication of healable superhydrophobic and slippery surfaces. Adv. Mater. 2014, 26, 7358–7364. [Google Scholar] [CrossRef]
- American Society for Testing and Materials (ASTM). Standard Test Method for Air Permeability of Textile Fabrics; American Society for Testing and Materials (ASTM): Philadelphia, PA, USA, 2012. [Google Scholar]
- Mortazavi, M.; Nosonovsky, M. A model for diffusion-driven hydrophobic recovery in plasma treated polymers. Appl. Surf. Sci. 2012, 258, 6876–6883. [Google Scholar] [CrossRef]
- Oh, J.-H.; Moon, M.-W.; Park, C.H. Effect of crystallinity on the recovery rate of superhydrophobicity in plasma-nanostructured polymers. RSC Adv. 2020, 10, 10939–10948. [Google Scholar] [CrossRef]
- Lafuma, A.; Quéré, D. Superhydrophobic states. Nat. Mater. 2003, 2, 457–460. [Google Scholar] [CrossRef]
- Shelby, M.D.; Hill, A.J.; Burgar, M.I.; Wilkes, G.L. The effects of molecular orientation on the physical aging and mobility of polycarbonate—Solid state NMR and dynamic mechanical analysis. J. Polym. Sci. Part B 2001, 39, 32–46. [Google Scholar] [CrossRef]
- Erbil, H.Y.; Demirel, A.L.; Avcı, Y.; Mert, O. Transformation of a simple plastic into a superhydrophobic surface. Science 2003, 299, 1377–1380. [Google Scholar] [CrossRef] [PubMed]
- Blossey, R. Self-cleaning surfaces—Virtual realities. Nat. Mater. 2003, 2, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.-J.; Kim, D.-H.; Im, M.; Lee, J.-H.; Yoon, J.-B.; Choi, Y.-K. “Lock-and-Key” geometry effect of patterned surfaces: Wettability and switching of adhesive force. Small 2009, 5, 90–94. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-M.; Yang, S. Directed water shedding on high-aspect-ratio shape memory polymer micropillar arrays. Adv. Mater. 2014, 26, 1283–1288. [Google Scholar] [CrossRef]






| Sample Codes | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Etching time (min) | E1 | E3 | E5 | E7 | E10 | E15 | |||||||||||||||
| Thermal aging Temperature (°C) | H120 | H120 | H100 | H110 | H120 | H120 | H40 | H60 | H80 | H100 | H110 | H120 | H100 | H110 | H120 | ||||||
| Thermal aging time (h) | 24 | 24 | 1 | 3 | 5 | 9 | 15 | 24 | 24 | 1 | 3 | 5 | 9 | 15 | 24 | 1 | 3 | 5 | 9 | 15 | 24 |
| Specimen Codes | Atomic Composition (at.%) | |||
|---|---|---|---|---|
| C | O | Total | ||
| UT | 100 | 0 | 100 | |
| E5 | 77.80 | 22.20 | 100 | |
| E10 | 73.52 | 26.48 | 100 | |
| E15 | 69.97 | 30.03 | 100 | |
| E5H120 1 h | 95.52 | 4.48 | 100 | |
| E10H120 1 h | 84.48 | 15.52 | 100 | |
| E15120 | 1 h | 78.50 | 21.50 | 100 |
| 5 h | 84.48 | 15.52 | 100 | |
| 24 h | 100 | 0 | 100 | |
| Weave Density (per in) | |||
|---|---|---|---|
| 62 × 62 | 72 × 72 | 82 × 80 | |
| WCA (°) | 155.2 ± 1.6 | 162.7 ± 2.4 | 166.3 ± 6.2 |
| ShA (°) | 8.4 ± 1.0 | 5.2 ± 0.7 | 2.2 ± 0.7 |
| SA (°) | >90 | >90 | >90 |
| Weave Density (per in) | |||
|---|---|---|---|
| 62 × 62 | 62 × 62 | 62 × 62 | |
| WCA (°) | 179.3 ± 2.3 | 180 ± 0.0 | 180 ± 0.0 |
| ShA (°) | 2.8 ± 0.4 | 1.8 ± 0.2 | 1.6 ± 0.2 |
| SA (°) | 11.2 ± 2.9 | 9.8 ± 2.3 | 4.2 ± 0.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, S.; Oh, J.-H.; Park, C.H. Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging. Polymers 2020, 12, 2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112756
Kim S, Oh J-H, Park CH. Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging. Polymers. 2020; 12(11):2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112756
Chicago/Turabian StyleKim, Shinyoung, Ji-Hyun Oh, and Chung Hee Park. 2020. "Development of Energy-Efficient Superhydrophobic Polypropylene Fabric by Oxygen Plasma Etching and Thermal Aging" Polymers 12, no. 11: 2756. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112756