Rheological and Mechanical Properties of Silica/Nitrile Butadiene Rubber Vulcanizates with Eco-Friendly Ionic Liquid
Abstract
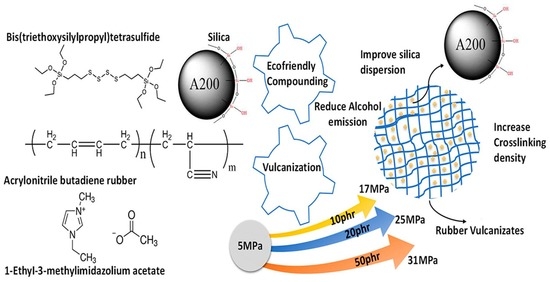
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Samples Preparation
2.3. Crosslinking Density
2.4. Transmission Electron Microscope
2.5. Scanning Electron Micrographs
2.6. Mechanical Properties
2.7. Rheological Measurements
3. Results and Discussion
3.1. Crosslinking Density and Vulcanization Dynamics
3.2. Dispersion of Silica and Other Additives
3.3. Thermal Properties
3.4. Tensile Properties
3.5. Rheological Behaviours
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- El-Nemr, K.F. Effect of different curing systems on the mechanical and physico-chemical properties of acrylonitrile butadiene rubber vulcanizates. Mater. Des. 2011, 32, 3361–3369. [Google Scholar] [CrossRef]
- Hagita, K.; Morita, H. Effects of polymer/filler interactions on glass transition temperatures of filler-filled polymer nanocomposites. Polymer 2019, 178, 121615. [Google Scholar] [CrossRef]
- Zheng, Z.; Song, Y.-H.; Zheng, Q. Interfacial structure and rheology of fumed silica filled polar oligomer nanocomposites. Acta Polym. Sin. 2017, 3, 429–453. [Google Scholar]
- Song, Y.; Zheng, Q. A guide for hydrodynamic reinforcement effect in nanoparticle-filled polymers. Crit. Rev. Solid State Mater. Sci. 2016, 41, 318–346. [Google Scholar] [CrossRef]
- García, B.F.; Saraji, S. Linear rheology of nanoparticle-enhanced viscoelastic surfactants. J. Mol. Liq. 2020, 300, 112215. [Google Scholar] [CrossRef]
- Stockelhuber, K.; Svistkov, A.; Pelevin, A.; Heinrich, G. Impact of filler surface modification on large scale mechanics of styrene butadiene/silica rubber composites. Macromolecules 2011, 44, 4366–4381. [Google Scholar] [CrossRef]
- Song, Y.; Yu, J.; Dai, D.; Song, L.; Jiang, N. Effect of silica particles modified by in-situ and ex-situ methods on the reinforcement of silicone rubber. Mater. Des. 2014, 64, 687–693. [Google Scholar] [CrossRef]
- Kaewsakul, W.; Sahakaro, K.; Dierkes, W.K.; Noordermeer, J.W. Mechanistic aspects of silane coupling agents with different functionalities on reinforcement of silica-filled natural rubber compounds. Polym. Eng. Sci. 2015, 55, 836–842. [Google Scholar] [CrossRef]
- Wu, F.; Zhang, S.; Chen, Z.; Zhang, B.; Yang, W.; Liu, Z.; Yang, M. Interfacial relaxation mechanisms in polymer nanocomposites through the rheological study on polymer/grafted nanoparticles. Polymer 2016, 90, 264–275. [Google Scholar] [CrossRef]
- Chen, Z.; Li, L.; Xiong, Z. Investigation on the interfacial behaviour between the rubber-cement matrix of the rubberized concrete. J. Clean. Prod. 2019, 209, 1354–1364. [Google Scholar] [CrossRef]
- Galindo-Rosales, F.J.; Moldenaers, P.; Vermant, J. Assessment of the dispersion quality in polymer nanocomposites by rheological methods. Macromol. Mater. Eng. 2011, 296, 331–340. [Google Scholar] [CrossRef] [Green Version]
- Hobbie, E.K. Shear rheology of carbon nanotube suspensions. Rheol. Acta 2010, 49, 323–334. [Google Scholar] [CrossRef]
- Harwood, J.; Payne, A.; Whittaker, R. Stress-softening and reinforcement of rubber. J. Macromol. Sci. Part B Phys. 1971, 5, 473–486. [Google Scholar] [CrossRef]
- Harwood, J.; Mullins, L.; Payne, A.R. Stress softening in natural rubber vulcanizates. Part II. Stress softening effects in pure gum and filler loaded rubbers. J. Appl. Polym. Sci. 1965, 9, 3011–3021. [Google Scholar] [CrossRef]
- Medalia, A.I. Electrical conduction in carbon black composites. Rubber Chem. Technol. 1986, 59, 432–454. [Google Scholar] [CrossRef]
- Merabia, S.; Sotta, P.; Long, D.R.J.M. A microscopic model for the reinforcement and the nonlinear behavior of filled elastomers and thermoplastic elastomers (Payne and Mullins effects). Macromolecules 2008, 41, 8252–8266. [Google Scholar] [CrossRef]
- Araujo-Morera, J.; Hernández Santana, M.; Verdejo, R.; López-Manchado, M.A. Giving a Second opportunity to tire waste: An alternative path for the development of sustainable self-healing styrene–butadiene rubber compounds overcoming the magic triangle of tires. Polymers 2019, 11, 2122. [Google Scholar] [CrossRef] [Green Version]
- Capezza, A.; Andersson, R.L.; Ström, V.; Wu, Q.; Sacchi, B.; Farris, S.; Hedenqvist, M.S.; Olsson, R.T. Preparation and comparison of reduced graphene oxide and carbon nanotubes as fillers in conductive natural rubber for flexible electronics. ACS Omega 2019, 4, 3458–3468. [Google Scholar] [CrossRef]
- Zhang, C.; Tang, Z.; Guo, B.; Zhang, L. Significantly improved rubber-silica interface via subtly controlling surface chemistry of silica. Compos. Sci. Technol. 2018, 156, 70–77. [Google Scholar] [CrossRef]
- Mohammed, B.S.; Awang, A.B.; San Wong, S.; Nhavene, C.P. Properties of nano silica modified rubbercrete. J. Clean. Prod. 2016, 119, 66–75. [Google Scholar] [CrossRef]
- Tang, Z.; Huang, J.; Wu, X.; Guo, B.; Zhang, L.; Liu, F. Interface engineering toward promoting silanization by ionic liquid for high-performance rubber/silica composites. Ind. Eng. Chem. Res. 2015, 54, 10747–10756. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, G.; Thareja, P. Surface chemistry driven dynamic rheology, microstructure of fumed and colloidal silica particles in nematic liquid crystals. J. Mol. Liq. 2020, 314, 113623. [Google Scholar] [CrossRef]
- Shanmugharaj, A.; Bhowmick, A.K. Dynamic mechanical properties of styrene-butadiene rubber vulcanizate filled with electron beam modified surface-treated dual-phase filler. J. Appl. Polym. Sci. 2003, 88, 2992–3004. [Google Scholar] [CrossRef]
- Nah, C.; Huh, M.Y.; Rhee, J.M.; Yoon, T.H. Plasma surface modification of silica and its effect on properties of styrene–butadiene rubber compound. Polym. Int. 2002, 51, 510–518. [Google Scholar] [CrossRef]
- Jiang, J.; Wang, W.; Shen, H.; Wang, J.; Cao, J. Characterization of silica particles modified with γ-methacryloxypropyltrimethoxysilane. Appl. Surf. Sci. 2017, 397, 104–111. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Ha, S.-W.; Jeon, S.-M.; Yoo, D.W.; Chun, S.-H.; Sohn, B.-H.; Lee, J.-K. Fabrication of triacetylcellulose−SiO2 nanocomposites by surface modification of silica nanoparticles. Langmuir 2010, 26, 7555–7560. [Google Scholar] [CrossRef]
- Chen, L.; Jia, Z.; Tang, Y.; Wu, L.; Luo, Y.; Jia, D. Novel functional silica nanoparticles for rubber vulcanization and reinforcement. Compos. Sci. Technol. 2017, 144, 11–17. [Google Scholar] [CrossRef]
- Dohi, H.; Horiuchi, S. Locating a silane coupling agent in silica-filled rubber composites by EFTEM. Langmuir 2007, 23, 12344–12349. [Google Scholar] [CrossRef]
- Tan, J.; Cheng, H.; Wei, L.; Wei, C.; Xing, Y.; Gui, X. Using low-rank coal slime as an eco-friendly replacement for carbon black filler in styrene butadiene rubber. J. Clean. Prod. 2019, 234, 949–960. [Google Scholar] [CrossRef]
- Lung, C.Y.K.; Matinlinna, J.P. Aspects of silane coupling agents and surface conditioning in dentistry: An overview. Dent. Mater. 2012, 28, 467–477. [Google Scholar] [CrossRef]
- Hallett, J.P.; Welton, T. Room-temperature ionic liquids: Solvents for synthesis and catalysis. 2. Chem. Rev. 2011, 111, 3508–3576. [Google Scholar] [CrossRef]
- Singh, S.K.; Savoy, A.W.J.J.o.M.L. Ionic liquids synthesis and applications: An overview. J. Mol. Liq. 2020, 297, 112038. [Google Scholar] [CrossRef]
- Shakeel, A.; Mahmood, H.; Farooq, U.; Ullah, Z.; Yasin, S.; Iqbal, T.; Chassagne, C.; Moniruzzaman, M. Rheology of pure ionic liquids and their complex fluids: A review. ACS Sustain. Chem. Eng. 2019, 7, 13586–13626. [Google Scholar] [CrossRef] [Green Version]
- Lei, J.; Gao, Y.; Zhao, X.; Zhu, Y.; Zhang, C.; Ma, Y.; Zhao, K.; Du, F. The dilational rheology and splashing behavior of ionic liquid-type imidazolium Gemini surfactant solutions: Impact of alkyl chain length. J. Mol. Liq. 2019, 283, 725–735. [Google Scholar] [CrossRef]
- Green, M.D.; Long, T.E. Designing imidazole-based ionic liquids and ionic liquid monomers for emerging technologies. Polym. Rev. 2009, 49, 291–314. [Google Scholar] [CrossRef]
- Ghandi, K. A review of ionic liquids, their limits and applications. Green Sustain. Chem. 2014, 4, 44–53. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.; Wang, H.; Bi, S.; Xue, Y.; Xue, Z.; Liao, Y.; Zhou, X.; Xie, X.; Mai, Y. Enhanced ion transport in polymer–ionic liquid electrolytes containing ionic liquid-functionalized nanostructured carbon materials. Carbon 2015, 86, 86–97. [Google Scholar] [CrossRef]
- Maciejewska, M.; Zaborski, M. Ionic liquids as coagents for sulfur vulcanization of butadiene–styrene elastomer filled with carbon black. Polym. Bull. 2018, 75, 4499–4514. [Google Scholar] [CrossRef] [Green Version]
- Kreyenschulte, H.; Richter, S.; Götze, T.; Fischer, D.; Steinhauser, D.; Klüppel, M.; Heinrich, G. Interaction of 1-allyl-3-methyl-imidazolium chloride and carbon black and its influence on carbon black filled rubbers. Carbon 2012, 50, 3649–3658. [Google Scholar] [CrossRef]
- Laskowska, A.; Marzec, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. Effect of imidazolium ionic liquid type on the properties of nitrile rubber composites. Polym. Int. 2013, 62, 1575–1582. [Google Scholar] [CrossRef]
- Xu, H.; Tong, F.; Yu, J.; Wen, L.; Zhang, J.; He, J. A one-pot method to prepare transparent poly (methyl methacrylate)/montmorillonite nanocomposites using imidazolium-based ionic liquids. Polym. Int. 2012, 61, 1382–1388. [Google Scholar] [CrossRef]
- Zhao, L.; Li, Y.; Cao, X.; You, J.; Dong, W. Multifunctional role of an ionic liquid in melt-blended poly (methyl methacrylate)/multi-walled carbon nanotube nanocomposites. Nanotechnology 2012, 23, 255702. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.; Yasin, S.; Adnan Akram, M.; Xu, H.; Song, Y.; Zheng, Q. Influence of ionic liquids on structure and rheological behaviors of silica-filled butadiene rubber. Ind. Eng. Chem. Res. 2019, 58, 18205–18212. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner Jr, J. Statistical mechanics of cross-linked polymer networks I. Rubberlike elasticity. J. Chem. Phys. 1943, 11, 512–520. [Google Scholar] [CrossRef]
- Marzocca, A.; Garraza, A.R.; Mansilla, M. Evaluation of the polymer–solvent interaction parameter χ for the system cured polybutadiene rubber and toluene. Polym. Test. 2010, 29, 119–126. [Google Scholar] [CrossRef]
- Zhang, X.; Xue, X.; Jia, H.; Wang, J.; Ji, Q.; Xu, Z. Influence of ionic liquid on the polymer–filler coupling and mechanical properties of nano-silica filled elastomer. J. Appl. Polym. Sci. 2017, 134, 44478. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, L.; Zheng, Q. Reconsideration of the rheology of silica filled natural rubber compounds. J. Phys. Chem. B 2017, 121, 5867–5875. [Google Scholar] [CrossRef]
- Zhong, B.; Jia, Z.; Hu, D.; Luo, Y.; Guo, B.; Jia, D. Surface modification of halloysite nanotubes by vulcanization accelerator and properties of styrene-butadiene rubber nanocomposites with modified halloysite nanotubes. Appl. Surf. Sci. 2016, 366, 193–201. [Google Scholar] [CrossRef]
- Lungwitz, R.; Linder, T.; Sundermeyer, J.; Tkatchenko, I.; Spange, S. Synthesis of chemisorbed imidazolium and phosphonium cations by reaction of ionic liquid precursors with silica. Chem. Commun. 2010, 46, 5903–5905. [Google Scholar] [CrossRef]
- Marwanta, E.; Mizumo, T.; Nakamura, N.; Ohno, H. Improved ionic conductivity of nitrile rubber/ionic liquid composites. Polymer 2005, 46, 3795–3800. [Google Scholar] [CrossRef]
- Zhang, C.; Mi, X.; Tian, J.; Zhang, J.; Xu, T. Supported ionic liquid silica as curing agent for epoxy composites with improved mechanical and thermal properties. Polymers 2017, 9, 478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, S.-Y.; Feng, X.-Q.; Lauke, B.; Mai, Y.-W. Effects of particle size, particle/matrix interface adhesion and particle loading on mechanical properties of particulate–polymer composites. Compos. Part B Eng. 2008, 39, 933–961. [Google Scholar] [CrossRef]
- Szadkowski, B.; Marzec, A.; Zaborski, M. Effect of in situ silanization of multiwalled carbon nanotubes on the properties of NBR/MWCNT-OH composites. Polym. Plast. Technol. Mater. 2019, 58, 1327–1341. [Google Scholar] [CrossRef]
- Weng, P.; Tang, Z.; Guo, B. Effects of Alkalinity of Ionic Liquid on Catalyzed Silanization in Rubber/Silica Composites. Ind. Eng. Chem. Res. 2019, 58, 18654–18662. [Google Scholar] [CrossRef]
- Liu, J.; Gao, Y.; Cao, D.; Zhang, L.; Guo, Z. Nanoparticle dispersion and aggregation in polymer nanocomposites: Insights from molecular dynamics simulation. Langmuir 2011, 27, 7926–7933. [Google Scholar] [CrossRef]
- Blume, A.; Thibault-Starzyk, F. Deciphering the silica/silane reaction mechanism for the development of a new generation of low rolling resistance tires: Part 1–Characterization by in situ IR spectroscopy. Rubber Fibres Plast. Int. 2017, 12, 152–157. [Google Scholar]
- Xu, Y.; Xu, H.; Zheng, Q.; Song, Y. Influence of ionic liquid on glass transition, dynamic rheology, and thermal stability of poly (methyl methacrylate)/silica nanocomposites. J. Appl. Polym. Sci. 2019, 136, 48007. [Google Scholar] [CrossRef]
- Huang, M.; Tunnicliffe, L.B.; Thomas, A.G.; Busfield, J.J. The glass transition, segmental relaxations and viscoelastic behaviour of particulate-reinforced natural rubber. Eur. Polym. J. 2015, 67, 232–241. [Google Scholar] [CrossRef]
- Xu, H.; Xia, X.; Hussain, M.; Song, Y.; Zheng, Q. Linear and nonlinear rheological behaviors of silica filled nitrile butadiene rubber. Polymer 2018, 156, 222–227. [Google Scholar] [CrossRef]
- Taguet, A.; Cassagnau, P.; Lopez-Cuesta, J.-M. Structuration, selective dispersion and compatibilizing effect of (nano) fillers in polymer blends. Prog. Polym. Sci. 2014, 39, 1526–1563. [Google Scholar] [CrossRef]
- Ou, Y.C.; Yu, Z.Z.; Vidal, A.; Donnet, J. Effects of alkylation of silicas on interfacial interaction and molecular motions between silicas and rubbers. J. Appl. Polym. Sci. 1996, 59, 1321–1328. [Google Scholar] [CrossRef]
- Saha, T.; Bhowmick, A.K. Influence of nanofiller on thermal degradation resistance of hydrogenated nitrile butadiene rubber. Rubber Chem. Technol. 2019, 92, 263–285. [Google Scholar] [CrossRef]
- Gao, W.; Lu, J.; Song, W.; Hu, J.; Han, B. Solution mechanochemical approach for preparing high-dispersion SiO2-g-SSBR and the performance of modified silica/SSBR composites. Ind. Eng. Chem. Res. 2019, 58, 7146–7155. [Google Scholar] [CrossRef]
- Maciejewska, M.; Walkiewicz, F.; Zaborski, M. Novel ionic liquids as accelerators for the sulfur vulcanization of butadiene–styrene elastomer composites. Ind. Eng. Chem. Res. 2013, 52, 8410–8415. [Google Scholar] [CrossRef]
- Xia, X.; Wu, Z.; Wang, W.; Shangguan, Y.; Zheng, Q. A facile and environmentally friendly approach to fabricate hybrid crosslinked nitrile butadiene rubber with comprehensively improved mechanical performances by incorporating sacrificial ionic bonds. Polymer 2019, 161, 55–63. [Google Scholar] [CrossRef]
- Xu, H.; Fan, X.; Song, Y.; Zheng, Q. Reinforcement and Payne effect of hydrophobic silica filled natural rubber nanocomposites. Compos. Sci. Technol. 2020, 187, 107943. [Google Scholar] [CrossRef]
- Marzec, A.; Laskowska, A.; Boiteux, G.; Zaborski, M.; Gain, O.; Serghei, A. The impact of imidazolium ionic liquids on the properties of nitrile rubber composites. Eur. Polym. J. 2014, 53, 139–146. [Google Scholar] [CrossRef]
- Yang, S.; Tian, J.; Bian, X.; Wu, Y. High performance NBR/fly ash composites prepared by an environment-friendly method. Compos. Sci. Technol. 2020, 186, 107909. [Google Scholar] [CrossRef]
- Xu, Y.; Xu, H.; Zheng, Q.; Song, Y. Influence of ionic liquids on rheological behaviors of polyisoprene rubber/silica compounds. Polymer 2019, 183, 121898. [Google Scholar] [CrossRef]
- Fan, X.; Xu, H.; Zhang, Q.; Song, Y.; Zheng, Q. Insight into the weak strain overshoot of carbon black filled natural rubber. Polymer 2019, 167, 109–117. [Google Scholar] [CrossRef]
- Song, Y.; Zeng, L.; Zheng, Q. Understanding the reinforcement and dissipation of natural rubber compounds filled with hybrid filler composed of carbon black and silica. Chin. J. Polym. Sci. 2017, 35, 1436–1446. [Google Scholar] [CrossRef]
- Van de Walle, A.; Tricot, C.; Gerspacher, M. Modeling carbon black reinforcement in rubber compounds. Kautsch. Gummi Kunstst. 1996, 49, 172–179. [Google Scholar]
- Divoux, T.; Barentin, C.; Manneville, S. Stress overshoot in a simple yield stress fluid: An extensive study combining rheology and velocimetry. Soft Matter 2011, 7, 9335–9349. [Google Scholar] [CrossRef] [Green Version]









| Ingredients | Amounts (phr) a |
|---|---|
| Nitrile butadiene rubber (NBR) | 100 |
| Silica | 0, 10, 20, 30, 40, 50 |
| Si69 b or Si69/[EMIM]OAc c (1/1) | 0, 0.46, 0.83, 1.15, 1.43, 1.67 (10 wt% of silica) |
| Antioxidant 6PPD d | 1 |
| Zinc oxide | 2 |
| Steric acid | 2 |
| Sulphur | 2 |
| Accelerator CZ e | 1 |
| Coupling Agent | / | Si69 | Si69/[EMIM]OAc | |||||
|---|---|---|---|---|---|---|---|---|
| φ | 0 | 0.041 | 0.079 | 0.177 | 0.041 | 0.079 | 0.177 | |
| Strain at break (%) | 100 °C | 409 ± 143 | 409 ± 143 | 409 ± 143 | 409 ± 143 | 409 ± 143 | 409 ± 143 | 409 ± 143 |
| 60 °C | 572 ± 19 | 357 ± 19 | 369 ± 5 | 600 ± 43 | 252 ± 19 | 360 ± 32 | 563 ± 12 | |
| RT | 659 ± 48 | 540 ± 31 | 614 ± 18 | 566 ± 82 | 472 ± 19 | 512 ± 32 | 559 ± 47 | |
| Tensile strength (MPa) | 100 °C | 1.5 ± 0.2 | 2.4 ± 0.3 | 4.1 ± 0.2 | 8.5 ± 0.7 | 2.7 ± 0.1 | 5.0 ± 0.3 | 11.2 ± 1.0 |
| 60 °C | 2.5 ± 0.3 | 4.0 ± 0.3 | 5.1 ± 0.2 | 14.4 ± 1.3 | 4.3 ± 0.4 | 9.8 ± 1.6 | 19.6 ± 1.5 | |
| RT | 6.4 ± 0.8 | 14.1 ± 2.1 | 20.9 ± 0.9 | 25.5 ± 1.5 | 17.4 ± 1.9 | 25.1 ± 1.8 | 31.0 ± 1.3 | |
| Stress at 100% strain (MPa) | 100 °C | 0.89 ± 0.1 | 1.5 ± 0.1 | 1.6 ± 0.1 | 2.0 ± 0.1 | 2.36 ± 0.2 | 2.3 ± 0.2 | 3.2 ± 0.5 |
| 60 °C | 0.59 ± 0.09 | 1.24 ± 0.09 | 1.42 ± 0.1 | 2.42 ± 0.23 | 1.73 ± 0.24 | 2.15 ± 0.30 | 2.56 ± 0.20 | |
| RT | 0.85 ± 0.10 | 1.48 ± 0.14 | 1.97 ± 0.2 | 2.84 ± 0.16 | 2.22 ± 0.7 | 2.97 ± 0.6 | 4.22 ± 0.8 | |
| Stress at 300% strain (MPa) | 100 °C | 1.61 ± 0.16 | 2.64 ± 0.43 | 4.31 ± 0.14 | 6.23 ± 0.34 | 2.85 ± 0.42 | 5.37 ± 0.34 | 9.90 ± 0.70 |
| 60 °C | 1.43 ± 0.20 | 3.22 ± 0.30 | 4.14 ± 0.17 | 7.08 ± 0.46 | 4.67 ± 0.60 | 8.05 ± 0.80 | 8.64 ± 0.50 | |
| RT | 1.92 ± 0.30 | 4.58 ± 0.40 | 6.69 ± 0.34 | 11.2 ± 0.8 | 8.0 ± 0.2 | 11.5 ± 1.1 | 14.8 ± 0.9 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hussain, M.; Yasin, S.; Memon, H.; Li, Z.; Fan, X.; Akram, M.A.; Wang, W.; Song, Y.; Zheng, Q. Rheological and Mechanical Properties of Silica/Nitrile Butadiene Rubber Vulcanizates with Eco-Friendly Ionic Liquid. Polymers 2020, 12, 2763. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112763
Hussain M, Yasin S, Memon H, Li Z, Fan X, Akram MA, Wang W, Song Y, Zheng Q. Rheological and Mechanical Properties of Silica/Nitrile Butadiene Rubber Vulcanizates with Eco-Friendly Ionic Liquid. Polymers. 2020; 12(11):2763. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112763
Chicago/Turabian StyleHussain, Munir, Sohail Yasin, Hafeezullah Memon, Zhiyun Li, Xinpeng Fan, Muhammad Adnan Akram, Wanjie Wang, Yihu Song, and Qiang Zheng. 2020. "Rheological and Mechanical Properties of Silica/Nitrile Butadiene Rubber Vulcanizates with Eco-Friendly Ionic Liquid" Polymers 12, no. 11: 2763. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12112763