Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020
Abstract
:1. Introduction
- Which are the leading countries and institutions in antibacterial dental adhesive literature?
- Which are the most influential journals in antibacterial dental adhesive literature?
- Which are the most trending and cited publications of antibacterial dental adhesive literature?
- What are the authorship and collaboration research patterns of antibacterial dental adhesive researchers?
- What are emerging research themes/keywords in antibacterial dental adhesive literature?
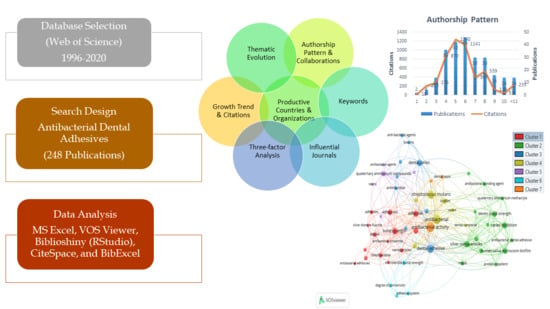
2. Methodology
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Analysis
2.4. Terms Used in Data Analysis
3. Results
3.1. Analysis of the Overall Growth Trend
3.2. Most Productive Countries and Organization on Antibacterial Dental Adhesive Research
3.3. Highly Influential Journals
3.4. Authorship Pattern
3.5. Authors’ Keyword Analyses on Antibacterial Dental Adhesive
3.6. Thematic Evolution Map of Author Keywords
3.7. Highly Cited Articles on Antibacterial Dental Adhesive
3.8. Three Factor (Sources, Countries, and Keywords) Relationships
3.9. Collaboration World Map of Dental Antibacterial Adhesive Literature
3.10. Citation Bursts of Dental Adhesive Literature
4. Discussion
4.1. Yearly Trend of Publications and Citations
4.2. Highly Productive and Cited Countries and Organizations
4.3. Highly Cited Papers
4.4. Preferred Journals
4.5. Frequently Used Keywords and Emerging Keywords (1996–2013, 2014–2016)
4.6. International Collaboration and Authorship Patterns
4.7. Limitations and Future Research Directions
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Bedran-Russo, A.; Leme-Kraus, A.A.; Vidal, C.M.; Teixeira, E.C. An Overview of Dental Adhesive Systems and the Dynamic Tooth–Adhesive Interface. Dent. Clin. 2017, 61, 713–731. [Google Scholar] [CrossRef] [PubMed]
- Florez, F.L.E.; Trofimov, A.A.; Ievlev, A.; Qian, S.; Rondinone, A.J.; Khajotia, S.S. Advanced characterization of surface-modified nanoparticles and nanofilled antibacterial dental adhesive resins. Sci. Rep. 2020, 10, 1–12. [Google Scholar]
- Sofan, E.; Sofan, A.; Palaia, G.; Tenore, G.; Romeo, U.; Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Van Meerbeek, B.; Yoshihara, K.; Van Landuyt, K.; Yoshida, Y.; Peumans, M. From Buonocore’s Pioneering Acid-Etch Technique to Self-Adhering Restoratives. A Status Perspective of Rapidly Advancing Dental Adhesive Technology. J. Adhes. Dent. 2020, 22, 7–34. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.S.; Syed, M.R. A review of bioceramics-based dental restorative materials. Dent. Mater. J. 2019, 38, 163–176. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, J.; Zhao, Y.; Tian, Z.; Zhu, J.; Shi, Z.; Cui, Z.; Zhu, S. Enhancement performance of application mussel-biomimetic adhesive primer for dentin adhesives. RSC Adv. 2020, 10, 12035–12046. [Google Scholar] [CrossRef] [Green Version]
- Khvostenko, D.; Hilton, T.; Ferracane, J.; Mitchell, J.; Kruzic, J. Bioactive glass fillers reduce bacterial penetration into marginal gaps for composite restorations. Dent. Mater. 2016, 32, 73–81. [Google Scholar] [CrossRef] [Green Version]
- Cadenaro, M.; Maravic, T.; Comba, A.; Mazzoni, A.; Fanfoni, L.; Hilton, T.; Ferracane, J.; Breschi, L. The role of polymerization in adhesive dentistry. Dent. Mater. 2019, 35, e1–e22. [Google Scholar] [CrossRef]
- Maroulakos, G.; He, J.; Nagy, W.W. The Post–endodontic Adhesive Interface: Theoretical Perspectives and Potential Flaws. J. Endod. 2018, 44, 363–371. [Google Scholar] [CrossRef] [Green Version]
- Yan, H.; Wang, S.; Han, L.; Peng, W.; Yi, L.; Guo, R.; Liu, S.; Yang, H.; Huang, C. Chlorhexidine-encapsulated mesoporous silica-modified dentin adhesive. J. Dent. 2018, 78, 83–90. [Google Scholar] [CrossRef]
- Tammaro, L.; Salle, A.D.; Calarco, A.; Luca, I.; Riccitiello, F.; Peluso, G.; Vittoria, V.; Sorrentino, A. Multifunctional Bioactive Resin for Dental Restorative Materials. Polymers 2020, 12, 332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiao, Y.; Tay, F.R.; Niu, L.-N.; Chen, J.-H. Advancing antimicrobial strategies for managing oral biofilm infections. Int. J. Oral Sci. 2019, 11, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chrószcz, M.; Barszczewska-Rybarek, I. Nanoparticles of Quaternary Ammonium Polyethylenimine Derivatives for Application in Dental Materials. Polymers 2020, 12, 2551. [Google Scholar] [CrossRef] [PubMed]
- Demarco, F.F.; Collares, K.; Correa, M.B.; Cenci, M.S.; Moraes, R.R.; Opdam, N.J. Should my composite restorations last forever? Why are they failing? Braz. Oral Res. 2017, 31, e56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.; Suh, B.; Yang, J. Antibacterial dental restorative materials: A review. Am. J. Dent. 2018, 31, 6B–12B. [Google Scholar]
- Ferrando-Magraner, E.; Bellot-Arcís, C.; Paredes-Gallardo, V.; Almerich-Silla, J.M.; García-Sanz, V.; Fernández-Alonso, M. Antibacterial properties of nanoparticles in dental restorative materials. A systematic review and meta-analysis. Medicina 2020, 56, 55. [Google Scholar] [CrossRef] [Green Version]
- Lung, C.Y.K.; Sarfraz, Z.; Habib, A.; Khan, A.S.; Matinlinna, J.P. Effect of silanization of hydroxyapatite fillers on physical and mechanical properties of a bis-GMA based resin composite. J. Mech. Behav. Biomed. Mater. 2016, 54, 283–294. [Google Scholar] [CrossRef]
- Hussain, N.; Khalid, H.; AlMaimouni, Y.K.; Ikram, S.; Khan, M.; Din, S.U.; Talal, A.; Khan, A.S. Microwave assisted urethane grafted nano-apatites for dental adhesives. J. Bioact. Compat. Polym. 2020, 35, 479–490. [Google Scholar] [CrossRef]
- Makvandi, P.; Jamaledin, R.; Jabbari, M.; Nikfarjam, N.; Borzacchiello, A. Antibacterial quaternary ammonium compounds in dental materials: A systematic review. Dent. Mater. 2018, 34, 851–867. [Google Scholar] [CrossRef]
- Imazato, S.; Ebi, N.; Takahashi, Y.; Kaneko, T.; Ebisu, S.; Russell, R.R. Antibacterial activity of bactericide-immobilized filler for resin-based restoratives. Biomaterials 2003, 24, 3605–3609. [Google Scholar] [CrossRef]
- Betancourt, D.; Baldion, P.; Castellanos, J.E. Resin-dentin bonding interface: Mechanisms of degradation and strategies for stabilization of the hybrid layer. Int. J. Biomater. 2019, 2019, 5268342. [Google Scholar] [CrossRef] [PubMed]
- Fujimura, Y.; Weerasinghe, D.; Kawashima, M. Development of an antibacterial bioactive dental adhesive: Simplicity and innovation. Am. J. Dent. 2018, 31, 13B–16B. [Google Scholar] [PubMed]
- Collares, F.M.; Garcia, I.M.; Klein, M.; Parolo, C.F.; Sánchez, F.A.L.; Takimi, A.; Bergmann, C.P.; Samuel, S.M.W.; Melo, M.A.; Leitune, V.C. Exploring Needle-Like Zinc Oxide Nanostructures for Improving Dental Resin Sealers: Design and Evaluation of Antibacterial, Physical and Chemical Properties. Polymers 2020, 12, 789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dias, P.G.; Da Silva, E.M.; Carvalho, C.; Miranda, M.; Portela, M.B.; Amaral, C.M. Characterization and Antibacterial Effect of an Experimental Adhesive Containing Different Concentrations of Proanthocyanidin. J. Adhes. Dent. 2020, 22, 139–147. [Google Scholar]
- Garcia, I.M.; Souza, V.S.; Scholten, J.D.; Collares, F.M. Quantum Dots of Tantalum Oxide with an Imidazolium Ionic Liquid as Antibacterial Agent for Adhesive Resin. J. Adhes. Dent. 2020, 22, 207–214. [Google Scholar]
- Cruzetta, L.; Garcia, I.M.; de Souza Balbinot, G.; Motta, A.S.; Collares, F.M.; Sauro, S.; CB Leitune, V. Evaluation of the Physicochemical and Antibacterial Properties of Experimental Adhesives Doped with Lithium Niobate. Polymers 2020, 12, 1330. [Google Scholar] [CrossRef]
- Cocco, A.R.; Da Rosa, W.L.d.O.; Da Silva, A.F.; Lund, R.G.; Piva, E. A systematic review about antibacterial monomers used in dental adhesive systems: Current status and further prospects. Dent. Mater. 2015, 31, 1345–1362. [Google Scholar] [CrossRef]
- Shafiei, F.; Memarpour, M. Antibacterial activity in adhesive dentistry: A literature review. Gen. Dent. 2012, 60, e346–e356. [Google Scholar]
- Shaikh, M.S.; Ullah, R.; Lone, M.A.; Matabdin, H.; Khan, F.; Zafar, M.S. Periodontal regeneration: A bibliometric analysis of the most influential studies. Regen. Med. 2019, 14, 1121–1136. [Google Scholar] [CrossRef]
- Tahim, A.; Bansal, H.; Goodson, A.M.C.; Payne, K.F.B.; Sabharwal, S. Open Access Publishing: A Study of Current Practice in Oral and Maxillofacial Surgery Research. J. Maxillofac. Oral Surg. 2016, 15, 517–520. [Google Scholar] [CrossRef] [Green Version]
- Tarazona, B.; Vidal-Infer, A.; Alonso-Arroyo, A. Bibliometric analysis of the scientific production in implantology (2009–2013). Clin. Oral Implants. Res. 2017, 28, 864–870. [Google Scholar] [CrossRef] [PubMed]
- Prevezanos, P.; Tsolakis, A.I.; Christou, P. Highly cited orthodontic articles from 2000 to 2015. Am. J. Orthod. Dentofac. Orthop. 2018, 153, 61–69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thornton, K.; Lee, D.J.; Yuan, J.C.-C.; Knoernschild, K.L.; Campbell, S.D.; Sukotjo, C. An Analysis of Prosthodontic Research Productivity: Geographic, Economic, and Collaborative Perspective. J. Prosthodont. 2012, 21, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Poletto, V.C.; Faraco Junior, I.M. Bibliometric study of articles published in a Brazilian journal of pediatric dentistry. Braz. Oral Res. 2010, 24, 83–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adnan, S.; Ullah, R. Top-cited articles in regenerative endodontics: A bibliometric analysis. J. Endod. 2018, 44, 1650–1664. [Google Scholar] [CrossRef]
- Ahn, S.J.; Lee, S.J.; Kook, J.K.; Lim, B.S. Experimental antimicrobial orthodontic adhesives using nanofillers and silver nanoparticles. Dent. Mater. 2009, 25, 206–213. [Google Scholar] [CrossRef]
- Ajami, A.A.; Rikhtegaran, S.; Bahari, M.; Hamadanchi, S. Antibacterial activity of self-adhesive resin cements against Streptococcus mutans at different time intervals. Iran. J. Microbiol. 2019, 11, 313–319. [Google Scholar] [CrossRef]
- Alghanem, A.; Fernandes, G.; Visser, M.; Dziak, R.; Renne, W.G.; Sabatini, C. Biocompatibility and bond degradation of poly-acrylic acid coated copper iodide-adhesives. Dent. Mater. 2017, 33, E336–E347. [Google Scholar] [CrossRef]
- Almaroof, A.; Niazi, S.A.; Rojo, L.; Mannocci, F.; Deb, S. Evaluation of dental adhesive systems incorporating an antibacterial monomer eugenyl methacrylate (EgMA) for endodontic restorations. Dent. Mater. 2017, 33, E239–E254. [Google Scholar] [CrossRef] [Green Version]
- Al-Musallam, T.A.; Evans, C.A.; Drummond, J.L.; Matasa, C.; Wu, C.D. Antimicrobial properties of an orthodontic adhesive combined with cetylpyridinium chloride. Am. J. Orthod. Dentofac. Orthop. 2006, 129, 245–251. [Google Scholar] [CrossRef]
- Al-Qarni, F.D.; Tay, F.; Weir, M.D.; Melo, M.A.S.; Sun, J.R.; Oates, T.W.; Xie, X.J.; Xu, H.H.K. Protein-repelling adhesive resin containing calcium phosphate nanoparticles with repeated ion-recharge and re-releases. J. Dent. 2018, 78, 91–99. [Google Scholar] [CrossRef] [PubMed]
- Altmann, A.S.P.; Collares, F.M.; Balbinot, G.D.; Leitune, V.C.B.; Takimi, A.S.; Samuel, S.M.W. Niobium pentoxide phosphate invert glass as a mineralizing agent in an experimental orthodontic adhesive. Angle Orthod. 2017, 87, 759–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, V.; Martinez, A.; Rojas, N.; Bello-Toledo, H.; Flores, P.; Sanchez-Sanhueza, G.; Catalan, A. Antibacterial activity against Streptococcus mutans and diametrical tensile strength of an interim cement modified with zinc oxide nanoparticles and terpenes: An in vitro study. J. Prosthet. Dent. 2018, 119, 7. [Google Scholar] [CrossRef] [PubMed]
- Andre, C.B.; Gomes, B.; Duque, T.M.; Rosalen, P.L.; Chan, D.C.N.; Ambrosano, G.M.B.; Giannini, M. Antimicrobial activity, effects on Streptococcus mutans biofilm and interfacial bonding of adhesive systems with and without antibacterial agent. Int. J. Adhes. Adhes. 2017, 72, 123–129. [Google Scholar] [CrossRef]
- Andre, C.B.; Rosalen, P.L.; Galvao, L.; Fronza, B.M.; Ambrosano, G.M.B.; Ferracane, J.L.; Giannini, M. Modulation of Streptococcus mutans virulence by dental adhesives containing anti-caries agents. Dent. Mater. 2017, 33, 1084–1092. [Google Scholar] [CrossRef]
- Andriani, A.; Purwanegara, M.K. Effect of titanium dioxide nanoparticle addition into orthodontic adhesive resin on enamel microhardness. In 1st Physics and Technologies in Medicine and Dentistry Symposium; IoP Publishing Ltd.: Bristol, UK, 2017; Volume 884. [Google Scholar]
- Arjmand, N.; Boruziniat, A.; Zakeri, M.; Mohammadipour, H.S. Microtensile bond strength of resin cement primer containing nanoparticles of silver (NAg) and amorphous calcium phosphate (NACP) to human dentin. J. Adv. Prosthodont. 2018, 10, 177–183. [Google Scholar] [CrossRef]
- Baena, E.; Cunha, S.R.; Maravic, T.; Comba, A.; Paganelli, F.; Alessandri-Bonetti, G.; Ceballos, L.; Tay, F.R.; Breschi, L.; Mazzoni, A. Effect of Chitosan as a Cross-Linker on Matrix Metalloproteinase Activity and Bond Stability with Different Adhesive Systems. Mar. Drugs 2020, 18, 263. [Google Scholar] [CrossRef]
- Bakhsh, T.A. Effect of Silver-Nitrate on interfacial gap detection under polymeric dental restoration in CP-OCT imaging. In Medical Imaging 2019: Biomedical Applications in Molecular, Structural, and Functional Imaging; Gimi, B., Krol, A., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2019; Volume 10953. [Google Scholar]
- Banzi, E.C.F.; Costa, A.R.; Puppin-Rontani, R.M.; Babu, J.; Garcia-Godoy, F. Inhibitory effects of a cured antibacterial bonding system on viability and metabolic activity of oral bacteria. Dent. Mater. 2014, 30, E238–E244. [Google Scholar] [CrossRef]
- Basting, R.T.; Goncalves, F.R.; Franca, F.M.G.; Do Amaral, F.L.B.; Florio, F.M. Antimicrobial Potential of Papain Chemomechanical Agent on Streptococcus Mutans and Lactobacillus Casei Followed by the Use of Self-Etching Adhesive Systems. J. Clin. Pediatr. Dent. 2016, 40, 62–68. [Google Scholar] [CrossRef]
- Bauer, J.; Silva, A.S.E.; Carvalho, E.M.; Carvalho, C.N.; Carvalho, R.M.; Manso, A.P. A niobophosphate bioactive glass suspension for rewetting dentin: Effect on antibacterial activity, pH and resin-dentin bonding durability. Int. J. Adhes. Adhes. 2018, 84, 178–183. [Google Scholar] [CrossRef]
- Bessudnova, N.O.; Bilenko, D.I.; Venig, S.B.; Atkin, V.S.; Zakharevich, A.M. A new X-ray adhesive system with embedded nano-particulate silver markers for dental applications. In Saratov Fall Meeting 2012: Optical Technologies in Biophysics and Medicine XIV; and Laser Physics and Photonics XIV; Tuchin, V.V., Genina, E.A., Derbov, V.L., Meglinski, I.V., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2013; Volume 8699. [Google Scholar]
- Bessudnova, N.O.; Bilenko, D.I.; Venig, S.B.; Shlyapnikova, O.A. Mechanical Properties of Adhesive System with a Silver Nanoparticulate Filler: An Experimental Study. In Saratov Fall Meeting 2013: Optical Technologies in Biophysics and Medicine XV; and Laser Physics and Photonics XV; Genina, E.A., Derbov, V.L., Meglinski, I., Tuchin, V.V., Eds.; Spie-Int Soc Optical Engineering: Bellingham, WA, USA, 2014; Volume 9031. [Google Scholar]
- Beyth, N.; Yudovin-Farber, I.; Bahir, R.; Domb, A.J.; Weissa, E.I. Antibacterial activity of dental composites containing quaternary ammonium polyethylenimine nanoparticles against Streptococcus mutans. Biomaterials 2006, 27, 3995–4002. [Google Scholar] [CrossRef] [PubMed]
- Bienek, D.R.; Giuseppetti, A.A.; Okeke, U.C.; Frukhtbeyn, S.A.; Dupree, P.J.; Khajotia, S.S.; Florez, F.L.E.; Hiers, R.D.; Skrtic, D. Antimicrobial, biocompatibility, and physicochemical properties of novel adhesive methacrylate dental monomers. J. Bioact. Compat. Polym. 2020, 35, 160–173. [Google Scholar] [CrossRef]
- Boutsiouki, C.; Franizenberger, R.; Lucker, S.; Kramer, N. Inhibition of secondary caries in vitro by addition of chlorhexidine to adhesive components. Dent. Mater. 2019, 35, 422–433. [Google Scholar] [CrossRef] [PubMed]
- Brambilla, E.; Ionescu, A.; Mazzoni, A.; Cadenaro, M.; Gagliani, M.; Ferraroni, M.; Tay, F.; Pashley, D.; Breschi, L. Hydrophilicity of dentin bonding systems influences in vitro Streptococcus mutans biofilm formation. Dent. Mater. 2014, 30, 926–935. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bregnocchi, A.; Zanni, E.; Uccelletti, D.; Marra, F.; Cavallini, D.; De Angelis, F.; De Bellis, G.; Bossu, M.; Ierardo, G.; Polimeni, A.; et al. Graphene-based dental adhesive with anti-biofilm activity. J. Nanobiotechnol. 2017, 15, 13. [Google Scholar] [CrossRef] [PubMed]
- Breschi, L.; Cammelli, F.; Visintini, E.; Mazzoni, A.; Vita, F.; Carrilho, M.; Cadenaro, M.; Foulger, S.; Mazzoti, G.; Tay, F.R.; et al. Influence of Chlorhexidine Concentration on the Durability of Etch-and-Rinse Dentin Bonds: A 12-month In Vitro Study. J. Adhes. Dent. 2009, 11, 191–198. [Google Scholar] [PubMed]
- Bridi, E.C.; Amaral, F.L.B.; Franca, F.M.G.; Turssi, C.P.; Florio, F.M.; Basting, R.T. In Vitro Effects of 2.5% Titanium Tetrafluoride on Streptococcus Mutans and Lactobacillus Casei in Dentin Followed by Self-Etching Adhesive Systems. Eur. J. Prosthodont. Restor. Dent. 2015, 23, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Bulut, H.; Turkun, M.; Turkun, L.S.; Isiksal, E. Evaluation of the shear bond strength of 3 curing bracket bonding systems combined with an antibacterial adhesive. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 77–83. [Google Scholar] [CrossRef]
- Cai, Y.L.; Stromme, M.; Melhus, A.; Engqvist, H.; Welch, K. Photocatalytic inactivation of biofilms on bioactive dental adhesives. J. Biomed. Mater. Res. Part B 2014, 102, 62–67. [Google Scholar] [CrossRef]
- Cal, E.; Turkun, L.S.; Turkun, M.; Toman, M.; Toksavul, S. Effect of an antibacterial adhesive on the bond strength of three different luting resin composites. J. Dent. 2006, 34, 372–380. [Google Scholar] [CrossRef]
- Cao, L.; Wu, J.L.; Zhang, Q.; Baras, B.; Bhadila, G.; Li, Y.C.; Melo, M.A.S.; Weir, M.D.; Bai, Y.X.; Zhang, N.; et al. Novel Protein-Repellent and Antibacterial Resins and Cements to Inhibit Lesions and Protect Teeth. Int. J. Polym. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Carvalho, F.G.; Carlo, H.L.; Sacramento, P.A.; De Barros, S.; Santos, R.L.; Puppin-Rontani, R.M. Biodegradation of caries-affected dentin bonding interface of fluoride and MDPB-containing adhesive system. Int. J. Adhes. Adhes. 2013, 47, 134–140. [Google Scholar] [CrossRef]
- Cehreli, Z.C.; Atac, A.S.; Sener, B. Antimicrobial properties of self-etching primer bonding systems. Oper. Dent. 2003, 28, 143–148. [Google Scholar] [PubMed]
- Chai, Z.; Li, F.; Fang, M.; Wang, Y.; Ma, S.; Xiao, Y.; Huang, L.; Chen, J. The bonding property and cytotoxicity of a dental adhesive incorporating a new antibacterial monomer. J. Oral Rehabil. 2011, 38, 849–856. [Google Scholar] [CrossRef]
- Chen, C.; Cheng, L.; Weir, M.D.; Lin, N.J.; Lin-Gibson, S.; Zhou, X.D.; Xu, H.H.K. Primer containing dimethylaminododecyl methacrylate kills bacteria impregnated in human dentin blocks. Int. J. Oral Sci. 2016, 8, 239–245. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Weir, M.D.; Cheng, L.; Lin, N.J.; Lin-Gibson, S.; Chow, L.C.; Zhou, X.D.; Xu, H.H.K. Antibacterial activity and ion release of bonding agent containing amorphous calcium phosphate nanoparticles. Dent. Mater. 2014, 30, 891–901. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Weir, M.D.; Zhang, K.; Arola, D.D.; Zhou, X.D.; Xu, H.H.K. Dental primer and adhesive containing a new antibacterial quaternary ammonium monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 345–355. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, K.; Melo, M.A.S.; Weir, M.D.; Zhou, X.; Xu, H.H.K. Anti-biofilm Dentin Primer with Quaternary Ammonium and Silver Nanoparticles. J. Dent. Res. 2012, 91, 598–604. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, K.; Weir, M.D.; Liu, H.B.; Zhou, X.D.; Xu, H.H.K. Effects of antibacterial primers with quaternary ammonium and nano-silver on Streptococcus mutans impregnated in human dentin blocks. Dent. Mater. 2013, 29, 462–472. [Google Scholar] [CrossRef] [Green Version]
- Cheng, L.; Zhang, K.; Zhang, N.; Melo, M.A.S.; Weir, M.D.; Zhou, X.D.; Bai, Y.X.; Reynolds, M.A.; Xu, H.H.K. Developing a New Generation of Antimicrobial and Bioactive Dental Resins. J. Dent. Res. 2017, 96, 855–863. [Google Scholar] [CrossRef]
- Cheng, L.; Zhang, K.; Zhou, C.C.; Weir, M.D.; Zhou, X.D.; Xu, H.H.K. One-year water-ageing of calcium phosphate composite containing nano-silver and quaternary ammonium to inhibit biofilms. Int. J. Oral Sci. 2016, 8, 172–181. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.R.; Da Rosa, W.L.D.; Peralta, S.L.; Maske, T.T.; da Silva, A.F.; Hartwig, C.A.; Mesko, M.F.; Piva, E.; Lund, R.G. New adhesive system based in metals cross-linking methacrylate. J. Mech. Behav. Biomed. Mater. 2018, 77, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Cocco, A.R.; Maske, T.T.; Lund, R.G.; Moraes, R.R. The antibacterial and physicochemical properties of a one-step dental adhesive modified with potential antimicrobial agents. Int. J. Adhes. Adhes. 2016, 71, 74–80. [Google Scholar] [CrossRef]
- Costa, J.F.; Siqueira, W.L.; Loguercio, A.D.; Reis, A.; De Oliveira, E.; Alves, C.M.C.; Bauer, J.R.D.; Grande, R.H.M. Characterization of aqueous silver nitrate solutions for leakage tests. J. Appl. Oral Sci. 2011, 19, 254–259. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, B.; Franca, F.M.G.; Florio, F.M.; Basting, R.T. In situ anticariogenic effect of adhesive systems containing fluoride and MDPB. Am. J. Dent. 2010, 23, 75–80. [Google Scholar]
- de Carvalho, F.G.; Puppin-Rontani, J.; Dos Santos, R.L.; Carlo, H.L.; Bonan, P.R.F.; Garcia-Godoy, F.; Puppin-Rontani, R.M. In vitro effect of S-mutans biofilm on fluoride/MDPB-containing adhesive system bonded to caries-affected primary dentin. Am. J. Dent. 2014, 27, 227–232. [Google Scholar]
- De Carvalho, F.G.; Puppin-Rontani, R.M.; De Fucio, S.B.P.; Negrini, T.D.; Carlo, H.L.; Garcia-Godoy, F. Analysis by confocal laser scanning microscopy of the MDPB bactericidal effect on S. mutans biofilm CLSM analysis of MDPB bactericidal effect on biofilm. J. Appl. Oral Sci. 2012, 20, 568–575. [Google Scholar] [CrossRef]
- De Siqueira, F.S.F.; Morales, L.A.R.; Granja, M.C.P.; De Melo, B.D.; Monteiro-Neto, V.; Reis, A.; Cardenas, A.F.M.; Loguercio, A.D. Effect of Silver Diamine Fluoride on the Bond in Properties to Caries-affected Dentin. J. Adhes. Dent. 2020, 22, 161–172. [Google Scholar] [CrossRef]
- Decha, N.; Talungchi, S.; Iawsipo, P.; Pikuingam, A.; Saiprasert, P.; Tansakul, C. Synthesis and characterization of new hydrolytic-resistant dental resin adhesive monomer HMTAF. Des. Monomers Polym. 2019, 22, 106–113. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Altmann, A.S.P.; Ferreira, C.J.; Arthur, R.A.; Leitune, V.C.B.; Samuel, S.M.W.; Collares, F.M. Evaluation of an antibacterial orthodontic adhesive incorporated with niobium-based bioglass: An in situ study. Braz. Oral Res. 2019, 33, 11. [Google Scholar] [CrossRef]
- Degrazia, F.W.; Leitune, V.C.B.; Garcia, I.M.; Arthur, R.A.; Samuel, S.M.W.; Collares, F.M. Effect of silver nanoparticles on the physicochemical and antimicrobial properties of an orthodontic adhesive. J. Appl. Oral Sci. 2016, 24, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Delauiz, Y.; Liu, T.W.; Deonarain, A.R.; Finer, Y.; Sholzati, B.; Santerre, J.P. Physical properties and cytotoxicity of antimicrobial dental resin adhesives containing dimethacrylate oligomers of Ciprofloxacin and Metronidazole. Dent. Mater. 2019, 35, 229–243. [Google Scholar] [CrossRef] [PubMed]
- Demirel, G.; Eryilmaz, M.; Seberol, H.; Gur, G. In vitro antibacterial activity of self-etch bio-active dental adhesives after artificial aging. Eur. Oral Res. 2019, 53, 32–37. [Google Scholar] [CrossRef] [PubMed]
- Deng, D.; Huang, X.; Huang, C.; Yang, T.; Du, X.; Wang, Y.; Ouyang, X.; Pei, D. Effects of chlorhexidine on bonding durability of different adhesive systems using a novel thermocycling method. Aust. Dent. J. 2013, 58, 148–155. [Google Scholar] [CrossRef]
- Deng, S.; Chung, K.H.; Chan, D.C.N.; Spiekerman, C. Evaluation of Bond Strength and Microleakage of a Novel Metal-titanate Antibacterial Agent. Oper. Dent. 2016, 41, E48–E56. [Google Scholar] [CrossRef] [Green Version]
- dos Santos, A.; Andre, C.B.; Martim, G.C.; Schuquel, I.T.A.; Pfeifer, C.S.; Ferracane, J.L.; Tominaga, T.T.; Khalil, N.M.; Radovanovic, E.; Girotto, E.M. Methacrylate saccharide-based monomers for dental adhesive systems. Int. J. Adhes. Adhes. 2018, 87, 1–11. [Google Scholar] [CrossRef]
- Du, X.J.; Huang, X.Q.; Huang, C.; Wang, Y.K.; Zhang, Y.F. Epigallocatechin-3-gallate (EGCG) enhances the therapeutic activity of a dental adhesive. J. Dent. 2012, 40, 485–492. [Google Scholar] [CrossRef]
- Dutra-Correa, M.; Leite, A.; de Cara, S.; Diniz, I.M.A.; Marques, M.M.; Suffredini, I.B.; Fernandes, M.S.; Toma, S.H.; Araki, K.; Medeiros, I.S. Antibacterial effects and cytotoxicity of an adhesive containing low concentration of silver nanoparticles. J. Dent. 2018, 77, 66–71. [Google Scholar] [CrossRef]
- Elsaka, S.E. Antibacterial activity and adhesive properties of a chitosan-containing dental adhesive. Quintessence Int. 2012, 43, 603–613. [Google Scholar]
- Eminkahyagil, N.; Korkmaz, Y.; Gokalp, S.; Baseren, M. Shear bond strength of orthodontic brackets with newly developed antibacterial self-etch adhesive. Angle Orthod. 2005, 75, 843–848. [Google Scholar]
- Feitosa, S.A.; Palasuk, J.; Kamocki, K.; Geraldeli, S.; Gregory, R.L.; Platt, J.A.; Windsor, L.J.; Bottino, M.C. Doxycycline-Encapsulated Nanotube-Modified Dentin Adhesives. J. Dent. Res. 2014, 93, 1270–1276. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, X.D.; Zhang, N.; Xu, H.H.K.; Weir, M.D.; Melo, M.A.S.; Bai, Y.X.; Zhang, K. Novel orthodontic cement containing dimethylaminohexadecyl methacrylate with strong antibacterial capability. Dent. Mater. J. 2017, 36, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Fernandes, G.; Renne, W.G.; Visser, M.B.; Sabatini, C. Esterase Inhibition and Copper Release from Copper Iodide Dental Adhesives—An In Vitro Study. J. Adhes. Dent. 2020, 22, 265–274. [Google Scholar] [CrossRef] [PubMed]
- Feuerstein, O.; Matalon, S.; Slutzky, H.; Weiss, E.I. Antibacterial properties of self-etching dental adhesive systems. J. Am. Dent. Assoc. 2007, 138, 349–354. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fik, C.P.; Konieczny, S.; Pashley, D.H.; Waschinski, C.J.; Ladisch, R.S.; Salz, U.; Bock, T.; Tiller, J.C. Telechelic Poly(2-oxazoline)s with a Biocidal and a Polymerizable Terminal as Collagenase Inhibiting Additive for Long-Term Active Antimicrobial Dental Materials. Macromol. Biosci. 2014, 14, 1569–1579. [Google Scholar] [CrossRef]
- Florez, F.L.E.; Hiers, R.D.; Larson, P.; Johnson, M.; O’Rear, E.; Rondinone, A.J.; Khajotia, S.S. Antibacterial dental adhesive resins containing nitrogen -doped titanium dioxide nanoparticles. Mater. Sci. Eng. C Mater. Biol. Appl. 2018, 93, 931–943. [Google Scholar] [CrossRef]
- Florez, F.L.E.; Hiers, R.D.; Zhao, Y.; Merritt, J.; Rondinone, A.J.; Khajotia, S.S. Optimization of a real-time high-throughput assay for assessment of Streptococcus mutans metabolism and screening of antibacterial dental adhesives. Dent. Mater. 2020, 36, 353–365. [Google Scholar] [CrossRef]
- Frolov, G.A.; Karasenkov, Y.N.; Gusev, A.A.; Zakharova, O.V.; Godymchuk, A.Y.; Kuznetsov, D.V.; Latuta, N.V.; Leont’ev, V.K. Germicidal Adhesives with Nanoparticles of Metals for Prevention of Recurrence of Caries. Nano Hybrids Compos. 2017, 13, 39–46. [Google Scholar] [CrossRef]
- Fu, D.J.; Lu, Y.; Gao, S.H.; Peng, Y.J.; Duan, H.Y. Chemical Property and Antibacterial Activity of Metronidazole-decorated Ti through Adhesive Dopamine. J. Wuhan Univ. Technol. Mater. Sci. Ed. 2019, 34, 968–972. [Google Scholar] [CrossRef]
- Fugolin, A.P.; Dobson, A.; Huynh, V.; Mbiya, W.; Navarro, O.; Franca, C.M.; Logan, M.; Merritt, J.L.; Ferracane, J.L.; Pfeifer, C.S. Antibacterial, ester-free monomers: Polymerization kinetics, mechanical properties, biocompatibility and anti-biofilm activity. Acta Biomater. 2019, 100, 132–141. [Google Scholar] [CrossRef]
- Gao, L.; Xie, X.J.; Wang, B.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Zhang, N.; Bai, Y.X. Protein-repellent and antibacterial effects of a novel polymethyl methacrylate resin. J. Dent. 2018, 79, 39–45. [Google Scholar] [CrossRef]
- Garcia, I.M.; Ferreira, C.J.; De Souza, V.S.; Leitune, V.C.B.; Samuel, S.M.W.; Balbinot, G.D.; Da Motta, A.D.; Visioli, F.; Scholten, J.D.; Collares, F.M. Ionic liquid as antibacterial agent for an experimental orthodontic adhesive. Dent. Mater. 2019, 35, 1155–1165. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Leitune, V.C.B.; Visioli, F.; Samuel, S.M.W.; Collares, F.M. Influence of zinc oxide quantum dots in the antibacterial activity and cytotoxicity of an experimental adhesive resin. J. Dent. 2018, 73, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Garcia, I.M.; Rodrigues, S.B.; Balbinot, G.D.; Visioli, F.; Leitune, V.C.B.; Collares, F.M. Quaternary ammonium compound as antimicrobial agent in resin-based sealants. Clin. Oral Investig. 2020, 24, 777–784. [Google Scholar] [CrossRef] [PubMed]
- Ge, Y.; Ren, B.; Zhou, X.D.; Xu, H.H.K.; Wang, S.P.; Li, M.Y.; Weir, M.D.; Feng, M.Y.; Cheng, L. Novel Dental Adhesive with Biofilm-Regulating and Remineralization Capabilities. Materials 2017, 10, 26. [Google Scholar] [CrossRef] [Green Version]
- Genari, B.; Leitune, V.C.B.; Jornada, D.S.; Aldrigui, B.R.; Pohlmann, A.R.; Guterres, S.S.; Samuel, S.M.W.; Collares, F.M. Effect on adhesion of a nanocapsules-loaded adhesive system. Braz. Oral Res. 2018, 32, 7. [Google Scholar] [CrossRef] [Green Version]
- Genari, B.; Leitune, V.C.B.; Jornada, D.S.; Camassola, M.; Arthur, R.A.; Pohlmann, A.R.; Guterres, S.S.; Collares, F.M.; Samuel, S.M.W. Antimicrobial effect and physicochemical properties of an adhesive system containing nanocapsules. Dent. Mater. 2017, 33, 735–742. [Google Scholar] [CrossRef]
- Geraldeli, S.; Soares, E.F.; Alvarez, A.J.; Farivar, T.; Shields, R.C.; Sinhoreti, M.A.C.; Nascimento, M.M. A new arginine-based dental adhesive system: Formulation, mechanical and anti-caries properties. J. Dent. 2017, 63, 72–80. [Google Scholar] [CrossRef]
- Giammanco, G.M.; Cumbo, E.M.G.; Luciani, A.; Gallina, G.; Mammina, C.; Pizzo, G. In vitro evaluation of the antibacterial activity of cured dentin/enamel adhesive incorporating the antimicrobial agent MDPB. New Microbiol. 2009, 32, 385–390. [Google Scholar]
- Godoy-Gallardo, M.; Mas-Moruno, C.; Fernandez-Calderon, M.C.; Perez-Giraldo, C.; Manero, J.M.; Albericio, F.; Gil, F.J.; Rodriguez, D. Covalent immobilization of hLf1-11 peptide on a titanium surface reduces bacterial adhesion and biofilm formation. Acta Biomater. 2014, 10, 3522–3534. [Google Scholar] [CrossRef]
- Gokcen, E.Y.; Oz, F.T.; Ozcelik, B.; Orhan, A.I.; Ozgul, B.M. Assessment of antibacterial activity of different treatment modalities in deciduous teeth: An in vitro study. Biotechnol. Biotechnol. Equip. 2016, 30, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- Gou, Y.P.; Li, J.Y.; Meghil, M.M.; Cutler, C.W.; Xu, H.H.K.; Tay, F.R.; Niu, L.N. Quaternary ammonium silane-based antibacterial and anti-proteolytic cavity cleanser. Dent. Mater. 2018, 34, 1814–1827. [Google Scholar] [CrossRef] [PubMed]
- Gou, Y.P.; Meghil, M.M.; Pucci, C.R.; Breschi, L.; Pashley, D.H.; Cutler, C.W.; Niu, L.N.; Li, J.Y.; Tay, F.R. Optimizing resin-dentin bond stability using a bioactive adhesive with concomitant antibacterial properties and anti-proteolytic activities. Acta Biomater. 2018, 75, 171–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gumustas, B.; Dosdogru, E.Y.; Guneysu, S. Evaluation of mechanical properties of fluoride-releasing dental materials after multiple fluoride recharge/discharge application. Biosci. J. 2020, 36, 1461–1470. [Google Scholar] [CrossRef]
- Guo, X.W.; Cheng, Q.L.; Yu, G.G.; Wang, H.; Tian, Z.L.; Shi, Z.S.; Cui, Z.C.; Zhu, S. The functions of hydrophobic elastic polyurethane combined with an antibacterial triclosan derivative in the dentin restoration interface. J. Mech. Behav. Biomed. Mater. 2020, 102, 9. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Malaquias, P.; Hass, V.; Matos, T.P.; Lourenco, L.; Reis, A.; Loguercio, A.D.; Farago, P.V. The role of copper nanoparticles in an etch-and-rinse adhesive on antimicrobial activity, mechanical properties and the durability of resindentine interfaces. J. Dent. 2017, 61, 12–20. [Google Scholar] [CrossRef]
- Gutierrez, M.F.; Malaquias, P.; Matos, P.; Szesz, A.; Souza, S.; Bermudez, J.; Reis, A.; Loguercio, A.D.; Farago, P.V. Mechanical and microbiological properties and drug release modeling of an etch-and-rinse adhesive containing copper nanoparticles. Dent. Mater. 2017, 33, 309–320. [Google Scholar] [CrossRef]
- Hafez, A.A.; Cox, C.F.; Tarim, B.; Otsuki, M.; Akimoto, N. An in vivo evaluation of hemorrhage control using sodium hypochlorite and direct capping with a one- or two-component adhesive system in exposed nonhuman primate pulps. Quintessence Int. 2002, 33, 261–272. [Google Scholar]
- Hamama, H.H.; Yiu, C.K.; Burrow, M.F. Effect of silver diamine fluoride and potassium iodide on residual bacteria in dentinal tubules. Aust. Dent. J. 2015, 60, 80–87. [Google Scholar] [CrossRef]
- Han, Q.; Li, B.L.; Zhou, X.D.; Ge, Y.; Wang, S.P.; Li, M.Y.; Ren, B.; Wang, H.H.; Zhang, K.K.; Xu, H.H.K.; et al. Anti-Caries Effects of Dental Adhesives Containing Quaternary Ammonium Methacrylates with Different Chain Lengths. Materials 2017, 10, 643. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, M.; Hirose, N.; Kitagawa, H.; Yamaguchi, S.; Imazato, S. Improving the durability of resin-dentin bonds with an antibacterial monomer MDPB. Dent. Mater. J. 2018, 37, 620–627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hashimoto, M.; Sasaki, J.I.; Yamaguchi, S.; Kawai, K.; Kawakami, H.; Iwasaki, Y.; Imazato, S. Gold Nanoparticles Inhibit Matrix Metalloproteases without Cytotoxicity. J. Dent. Res. 2015, 94, 1085–1091. [Google Scholar] [CrossRef] [PubMed]
- He, J.W.; Soderling, E.; Lassila, L.V.; Vallittu, P.K. Preparation of antibacterial and radio-opaque dental resin with new polymerizable quaternary ammonium monomer. Dent. Mater. 2015, 31, 575–582. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Weir, M.D.; Hsia, R.C.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesive containing antibacterial agents and calcium phosphate nanoparticles. J. Biomed. Mater. Res. Part B 2013, 101B, 620–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Herrera, M.; Castillo, A.; Bravo, M.; Liebana, J.; Carrion, P. Antibacterial activity of resin adhesives, glass ionomer and resin-modified glass ionomer cements and a compomer in contact with dentin caries samples. Oper. Dent. 2000, 25, 265–269. [Google Scholar] [PubMed]
- Hirose, N.; Kitagawa, R.; Kitagawa, H.; Maezono, H.; Mine, A.; Hayashi, M.; Haapasalo, M.; Imazato, S. Development of a Cavity Disinfectant Containing Antibacterial Monomer MDPB. J. Dent. Res. 2016, 95, 1487–1493. [Google Scholar] [CrossRef]
- Huang, B.; Cuitizouitch, D.G.; Santerre, J.P.; Finer, Y. Biodegradation of resin-dentin interfaces is dependent on the restorative material, mode of adhesion, esterase or MMP inhibition. Dent. Mater. 2018, 34, 1253–1262. [Google Scholar] [CrossRef]
- Huang, L.; Xiao, Y.H.; Xing, X.D.; Li, F.; Ma, S.; Qi, L.L.; Chen, J.H. Antibacterial activity and cytotoxicity of two novel cross-linking antibacterial monomers on oral pathogens. Arch. Oral Biol. 2011, 56, 367–373. [Google Scholar] [CrossRef]
- Huang, L.; Yu, F.; Sun, X.; Dong, Y.; Lin, P.T.; Yu, H.H.; Xiao, Y.H.; Chai, Z.G.; Xing, X.D.; Chen, J.H. Antibacterial activity of a modified unfilled resin containing a novel polymerizable quaternary ammonium salt MAE-HB. Sci. Rep. 2016, 6, 8. [Google Scholar] [CrossRef]
- Ibrahim, M.S.; AlQarni, F.D.; Al-Dulaijan, Y.A.; Weir, M.D.; Oates, T.W.; Xu, H.H.K.; Melo, M.A.S. Tuning Nano-Amorphous Calcium Phosphate Content in Novel Rechargeable Antibacterial Dental Sealant. Materials 2018, 11, 1544. [Google Scholar] [CrossRef] [Green Version]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Ebisu, S.; Tay, F.R. Antibacterial activity and bonding characteristics of an adhesive resin containing antibacterial monomer MDPB. Dent. Mater. 2003, 19, 313–319. [Google Scholar] [CrossRef]
- Imazato, S.; Kinomoto, Y.; Tarumi, H.; Torii, M.; Russell, R.R.B.; McCabe, J.F. Incorporation of antibacterial monomer MDPB into dentin primer. J. Dent. Res. 1997, 76, 768–772. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Kuramoto, A.; Takahashi, Y.; Ebisu, S.; Peters, M.C. In vitro antibacterial effects of the dentin primer of Clearfil Protect Bond. Dent. Mater. 2006, 22, 527–532. [Google Scholar] [CrossRef] [PubMed]
- Imazato, S.; Ma, S.; Chen, J.H.; Xu, H.H.K. Therapeutic polymers for dental adhesives: Loading resins with bio-active components. Dent. Mater. 2014, 30, 97–104. [Google Scholar] [CrossRef] [Green Version]
- Imazato, S.; Tay, F.R.; Kaneshiro, A.V.; Takahashi, Y.; Ebisu, S. An in vivo evaluation of bonding ability of comprehensive antibacterial adhesive system incorporating MDPB. Dent. Mater. 2007, 23, 170–176. [Google Scholar] [CrossRef]
- Imazato, S.; Walls, A.W.G.; Kuramoto, A.; Ebisu, S. Penetration of an antibacterial dentine-bonding system into demineralized human root dentine in vitro. Eur. J. Oral Sci. 2002, 110, 168–174. [Google Scholar] [CrossRef]
- Inagaki, L.T.; Alonso, R.C.B.; Araujo, G.A.S.; De Souza, E.J.C.; Anibal, P.C.; Hofling, J.F.; Pascon, F.M.; Puppin-Rontani, R.M. Effect of monomer blend and chlorhexidine-adding on physical, mechanical and biological properties of experimental infiltrants. Dent. Mater. 2016, 32, E307–E313. [Google Scholar] [CrossRef]
- Jaymand, M.; Lotfi, M.; Barar, J.; Kimyai, S. Synthesis and characterization of potential multifunctional methacrylate-based dental monomers. Res. Chem. Intermed. 2017, 43, 5707–5722. [Google Scholar] [CrossRef]
- Jiang, M.; Mei, M.L.; Wong, M.C.M.; Chu, C.H.; Lo, E.C.M. Effect of silver diamine fluoride solution application on the bond strength of dentine to adhesives and to glass ionomer cements: A systematic review. BMC Oral Health 2020, 20, 10. [Google Scholar] [CrossRef]
- Jo, J.K.; El-Fiqi, A.; Lee, J.H.; Kim, D.A.; Kim, H.W.; Lee, H.H. Rechargeable microbial anti-adhesive polymethyl methacrylate incorporating silver sulfadiazine-loaded mesoporous silica nanocarriers. Dent. Mater. 2017, 33, E361–E372. [Google Scholar] [CrossRef]
- Jung, J.H.; Kim, D.H.; Yoo, K.H.; Yoon, S.Y.; Kim, Y.; Bae, M.K.; Chung, J.; Ko, C.C.; Kwon, Y.H.; Kim, Y.I. Dentin sealing and antibacterial effects of silver-doped bioactive glass/mesoporous silica nanocomposite: An in vitro study. Clin. Oral Investig. 2019, 23, 253–266. [Google Scholar] [CrossRef] [PubMed]
- Kapdan, A.; Oztas, N. Effects of chlorhexidine and gaseous ozone on microleakage and on the bond strength of dentin bonding agents with compomer restoration on primary teeth. J. Dent. Sci. 2015, 10, 46–54. [Google Scholar] [CrossRef] [Green Version]
- Kim, C.S.; Ozer, F.; Mante, F.K. Fracture mechanics of dental adhesives supplemented with Polymethyl-vinyl-ether-co-maleic anhydride. J. Adhes. Sci. Technol. 2017, 31, 1116–1124. [Google Scholar] [CrossRef]
- Kim, H.J.; Kwon, T.Y.; Kim, K.H.; Kwon, S.T.; Cho, D.H.; Son, J.S. Long-term release of chlorhexidine from dental adhesive resin system using human serum albumin nanoparticles. Polym. Bull. 2014, 71, 875–886. [Google Scholar] [CrossRef]
- Kitagawa, R.; Kitagawa, H.; Izutani, N.; Hirose, N.; Hayashi, M.; Imazato, S. Development of an Antibacterial Root Canal Filling System Containing MDPB. J. Dent. Res. 2014, 93, 1277–1282. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, H.; Hamama, H.H.; Burrow, M.F. Effect of a silver diamine fluoride and potassium iodide-based desensitizing and cavity cleaning agent on bond strength to dentine. Int. J. Adhes. Adhes. 2016, 68, 54–61. [Google Scholar] [CrossRef]
- Konar, M.; Nayak, N.; Priyadarsini, S.; Mishra, M.; Sahoo, H. Antimicrobial activity of nanoparticle-based dental fillers on novel chromogenic bacteria Enterobacter ludwigii. Mater. Res. Express 2019, 6, 10. [Google Scholar] [CrossRef]
- Koulaouzidou, E.A.; Helvatjoglu-Antoniades, M.; Palaghias, G.; Karanika-Kouma, A.; Antoniades, D. Cytotoxicity evaluation of an antibacterial dentin adhesive system on established cell lines. J. Biomed. Mater. Res. Part B 2008, 84B, 271–276. [Google Scholar] [CrossRef]
- Kramer, N.; Mohwald, M.; Lucker, S.; Domann, E.; Zorzin, J.I.; Rosentritt, M.; Frankenberger, R. Effect of microparticulate silver addition in dental adhesives on secondary caries in vitro. Clin. Oral Investig. 2015, 19, 1673–1681. [Google Scholar] [CrossRef]
- Kuang, X.Y.; Chen, V.; Xu, X. Novel Approaches to the Control of Oral Microbial Biofilms. Biomed. Res. Int. 2018, 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Kwon, T.Y.; Hong, S.H.; Kim, Y.K.; Kim, K.H. Antibacterial Effects of 4-META/MMA-TBB Resin Containing Chlorhexidine. J. Biomed. Mater. Res. Part B 2010, 92B, 561–567. [Google Scholar] [CrossRef] [PubMed]
- Lapinska, B.; Konieczka, M.; Zarzycka, B.; Sokolowski, K.; Grzegorczyk, J.; Lukomska-Szymanska, M. Flow Cytometry Analysis of Antibacterial Effects of Universal Dentin Bonding Agents on Streptococcus mutans. Molecules 2019, 24, 532. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.J.; Heo, M.; Lee, D.; Han, S.; Moon, J.H.; Lim, H.N.; Kwon, I.K. Preparation and characterization of antibacterial orthodontic resin containing silver nanoparticles. Appl. Surf. Sci. 2018, 432, 317–323. [Google Scholar] [CrossRef]
- Lessa, F.C.R.; Nogueira, I.; Huck, C.; Hebling, J.; Costa, C.A.D. Transdentinal Cytotoxic Effects of Different Concentrations of Chlorhexidine Gel Applied on Acid-Conditioned Dentin Substrate. J. Biomed. Mater. Res. Part B 2010, 92B, 40–47. [Google Scholar] [CrossRef]
- Li, F.; Chai, Z.G.; Sun, M.N.; Wang, F.; Ma, S.; Zhang, L.; Fang, M.; Chen, J.H. Anti-biofilm Effect of Dental Adhesive with Cationic Monomer. J. Dent. Res. 2009, 88, 372–376. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Chen, J.H.; Chai, Z.G.; Zhang, L.; Xiao, Y.H.; Fang, M.; Ma, S. Effects of a dental adhesive incorporating antibacterial monomer on the growth, adherence and membrane integrity of Streptococcus mutans. J. Dent. 2009, 37, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Li, F.; Majd, H.; Weir, M.D.; Arola, D.D.; Xu, H.H.K. Inhibition of matrix metalloproteinase activity in human dentin via novel antibacterial monomer. Dent. Mater. 2015, 31, 284–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Effect of salivary pellicle on antibacterial activity of novel antibacterial dental adhesives using a dental plaque microcosm biofilm model. Dent. Mater. 2014, 30, 182–191. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Weir, M.D.; Chen, J.H.; Xu, H.H.K. Comparison of quaternary ammonium-containing with nano-silver-containing adhesive in antibacterial properties and cytotoxicity. Dent. Mater. 2013, 29, 450–461. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Weir, M.D.; Chen, J.H.; Xu, H.H.K. Effect of charge density of bonding agent containing a new quaternary ammonium methacrylate on antibacterial and bonding properties. Dent. Mater. 2014, 30, 433–441. [Google Scholar] [CrossRef] [Green Version]
- Li, F.; Weir, M.D.; Fouad, A.F.; Xu, H.H.K. Time-kill behaviour against eight bacterial species and cytotoxicity of antibacterial monomers. J. Dent. 2013, 41, 881–891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, F.; Weir, M.D.; Xu, H.H.K. Effects of Quaternary Ammonium Chain Length on Antibacterial Bonding Agents. J. Dent. Res. 2013, 92, 932–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Yu, X.L.; Liu, F.; Deng, F.L.; He, J.W. Synthesis of antibacterial dimethacrylate derived from niacin and its application in preparing antibacterial dental resin system. J. Mech. Behav. Biomed. Mater. 2020, 102, 7. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Qi, M.L.; Sun, X.L.; Chi, M.H.; Wan, Y.; Zheng, X.F.; Li, C.Y.; Wang, L.; Dong, B. Novel dental adhesive containing silver exchanged EMT zeolites against cariogenic biofilms to combat dental caries. Microporous Mesoporous Mater. 2020, 299, 10. [Google Scholar] [CrossRef]
- Li, Y.C.; Hu, X.Y.; Ruan, J.P.; Arola, D.D.; Ji, C.; Weir, M.D.; Oates, T.W.; Chang, X.F.; Zhang, K.; Xu, H.H.K. Bonding durability, antibacterial activity and biofilm pH of novel adhesive containing antibacterial monomer and nanoparticles of amorphous calcium phosphate. J. Dent. 2019, 81, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Hu, X.Y.; Xia, Y.; Ji, Y.D.; Ruan, J.P.; Weir, M.D.; Lin, X.Y.; Nie, Z.H.; Gu, N.; Masri, R.; et al. Novel magnetic nanoparticle-containing adhesive with greater dentin bond strength and antibacterial and remineralizing capabilities. Dent. Mater. 2018, 34, 1310–1322. [Google Scholar] [CrossRef]
- Liang, J.G.; Li, M.Y.; Ren, B.A.; Wu, T.M.; Xu, H.H.K.; Liu, Y.; Peng, X.; Yang, G.; Weir, M.D.; Zhang, S.Y.; et al. The anti-caries effects of dental adhesive resin influenced by the position of functional groups in quaternary ammonium monomers. Dent. Mater. 2018, 34, 400–411. [Google Scholar] [CrossRef]
- Liang, K.N.; Wang, S.P.; Tao, S.Y.; Xiao, S.M.; Zhou, H.; Wang, P.; Cheng, L.; Zhou, X.D.; Weir, M.D.; Oates, T.W.; et al. Dental remineralization via poly(amido amine) and restorative materials containing calcium phosphate nanoparticles. Int. J. Oral Sci. 2019, 11, 12. [Google Scholar] [CrossRef] [Green Version]
- Liang, X.X.; Huang, Q.T.; Liu, F.; He, J.W.; Lin, Z.M. Synthesis of novel antibacterial monomers (UDMQA) and their potential application in dental resin. J. Appl. Polym. Sci. 2013, 129, 3373–3381. [Google Scholar] [CrossRef]
- Liu, D.; Peng, X.; Wang, S.P.; Han, Q.; Li, B.L.; Zhou, X.X.; Ren, B.; Xu, H.H.K.; Weir, M.D.; Li, M.Y.; et al. A novel antibacterial resin-based root canal sealer modified by Dimethylaminododecyl Methacrylate. Sci. Rep. 2019, 9, 9. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, L.; Niu, L.N.; Yu, T.; Xu, H.H.K.; Weir, M.D.; Oates, T.W.; Tay, F.R.; Chen, J.H. Antibacterial and remineralizing orthodontic adhesive containing quaternary ammonium resin monomer and amorphous calcium phosphate nanoparticles. J. Dent. 2018, 72, 53–63. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.M.; Goncalves, R.B.; Pimenta, L.A.F.; Bedran-Russo, A.K.B.; Pereira, P.N.R. In vitro evaluation of caries inhibition promoted by self-etching adhesive systems containing antibacterial agents. J. Biomed. Mater. Res. Part B 2005, 75B, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Luo, W.X.; Huang, Q.T.; Liu, F.; Lin, Z.M.; He, J.W. Synthesis of antibacterial methacrylate monomer derived from thiazole and its application in dental resin. J. Mech. Behav. Biomed. Mater. 2015, 49, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Shan, L.Q.; Xiao, Y.H.; Li, F.; Huang, L.; Shen, L.J.; Chen, J.H. The cytotoxicity of methacryloxylethyl cetyl ammonium chloride, a cationic antibacterial monomer, is related to oxidative stress and the intrinsic mitochondrial apoptotic pathway. Braz. J. Med. Biol. Res. 2011, 44, 1125–1133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machado, A.H.S.; Garcia, I.M.; Da Motta, A.D.; Leitune, V.C.B.; Collares, F.M. Triclosan-loaded chitosan as antibacterial agent for adhesive resin. J. Dent. 2019, 83, 33–39. [Google Scholar] [CrossRef]
- Magro, M.G.; Kuga, M.C.; Victorino, K.R.; Vazquez-Garcia, F.A.; Aranda-Garcia, A.J.; Faria, N.B.; Faria, G.; Shinohara, A.L. Evaluation of the Interaction Between Sodium Hypochlorite and Several Formulations Containing Chlorhexidine and its Effect on the Radicular Dentin-SEM and Push-Out Bond Strength Analysis. Microsc. Res. Tech. 2014, 77, 17–22. [Google Scholar] [CrossRef]
- Maia, A.C.; Mangabeira, A.; Vieira, R.; Neves, A.D.; Lopes, R.T.; Pires, T.M.; Viana, G.M.; Cabral, L.M.; Cavalcante, L.M.; Portela, M.B. Experimental composites containing quaternary ammonium methacrylates reduce demineralization at enamel-restoration margins after cariogenic challenge. Dent. Mater. 2019, 35, E175–E183. [Google Scholar] [CrossRef]
- Manouchehri, F.; Sadeghi, B.; Najafi, F.; Mosslemin, M.H.; Niakan, M. Synthesis and characterization of novel polymerizable bis-quaternary ammonium dimethacrylate monomers with antibacterial activity as an efficient adhesive system for dental restoration. Polym. Bull. 2019, 76, 1295–1315. [Google Scholar] [CrossRef]
- Markham, M.D.; Tsujimoto, A.; Barkmeier, W.W.; Jurado, C.A.; Fischer, N.G.; Watanabe, H.; Baruth, A.G.; Latta, M.A.; Garcia-Godoy, F. Influence of 38% silver diamine fluoride application on bond stability to enamel and dentin using universal adhesives in self-etch mode. Eur. J. Oral Sci. 2020. [Google Scholar] [CrossRef]
- Martim, G.C.; Kupfer, V.L.; Moises, M.P.; Dos Santos, A.; Buzzetti, P.H.M.; Rinaldi, A.W.; Rubira, A.F.; Girotto, E.M. Physical-chemical properties of dental composites and adhesives containing silane-modified SBA-15. J. Mech. Behav. Biomed. Mater. 2018, 80, 277–284. [Google Scholar] [CrossRef]
- Matsuo, K.; Yoshihara, K.; Nagaoka, N.; Makita, Y.; Obika, H.; Okihara, T.; Matsukawa, A.; Yoshida, Y.; Van Meerbeek, B. Rechargeable anti-microbial adhesive formulation containing cetylpyridinium chloride montmorillonite. Acta Biomater. 2019, 100, 388–397. [Google Scholar] [CrossRef] [PubMed]
- Meiers, J.C.; Miller, G.A. Antibacterial activity of dentin bonding systems, resin modified glass ionomers, and polyacid modified composite resins. Oper. Dent. 1996, 21, 257–264. [Google Scholar] [PubMed]
- Melinte, V.; Buruiana, T.; Aldea, H.; Matiut, S.; Silion, M.; Buruiana, E.C. Photopolymerizable phosphate acrylates as comonomers in dental adhesives with or without triclosan monomer units. Mater. Sci. Eng. C-Mater. Biol. Appl. 2014, 34, 176–185. [Google Scholar] [CrossRef] [PubMed]
- Melinte, V.; Buruiana, T.; Chibac, A.; Mares, M.; Aldea, H.; Buruiana, E.C. New acid BisGMA analogs for dental adhesive applications with antimicrobial activity. Dent. Mater. 2016, 32, E314–E326. [Google Scholar] [CrossRef] [PubMed]
- Melo, M.A.S.; Cheng, L.; Zhang, K.; Weir, M.D.; Rodrigues, L.K.A.; Xu, H.H.K. Novel dental adhesives containing nanoparticles of silver and amorphous calcium phosphate. Dent. Mater. 2013, 29, 199–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Melo, M.A.S.; Weir, M.D.; Passos, V.F.; Rolim, J.P.M.; Lynch, C.D.; Rodrigues, L.K.A.; Xu, H.H.K. Human In Situ Study of the effect of Bis(2-Methacryloyloxyethyl) Dimethylammonium Bromide Immobilized in Dental Composite on Controlling Mature Cariogenic Biofilm. Int. J. Mol. Sci. 2018, 19, 3443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mestres, G.; Abdolhosseini, M.; Bowles, W.; Huang, S.H.; Aparicio, C.; Gorr, S.U.; Ginebra, M.P. Antimicrobial properties and dentin bonding strength of magnesium phosphate cements. Acta Biomater. 2013, 9, 8384–8393. [Google Scholar] [CrossRef]
- Mestres, G.; Ginebra, M.P. Novel magnesium phosphate cements with high early strength and antibacterial properties. Acta Biomater. 2011, 7, 1853–1861. [Google Scholar] [CrossRef]
- Minick, G.T.; Oesterle, L.J.; Newman, S.M.; Shellhart, W.C. Bracket bond strengths of new adhesive systems. Am. J. Orthod. Dentofac. Orthop. 2009, 135, 771–776. [Google Scholar] [CrossRef]
- Mocquot, C.; Cabrera, A.; Colon, P.; Bosco, J.; Grosgogeat, B.; Pradelle-Plasse, N. Effect of a hyperbaric environment (diving conditions) on adhesive restorations: An in vitro study. Br. Dent. J. 2017, 223, 347–351. [Google Scholar] [CrossRef]
- Monjaras-Avila, A.J.; Zavala-Alonso, N.V.; Martinez-Castanon, G.A.; Patino-Marin, N.; Flores, D.S.H.; Ruiz, F. Sodium Hypochlorite as Fluorotic Dentin Pretreatment of Two-Step Self-Etch Adhesive with Silver Nanoparticle: Atomic Force Microscope and Adhesive Microtensile Bond Strength Evaluation. J. Nanomater. 2017, 2017, 1381929. [Google Scholar] [CrossRef]
- Moreira, D.M.; Oei, J.; Rawls, H.R.; Wagner, J.; Chu, L.R.; Li, Y.M.; Zhang, W.; Whang, K. A novel antimicrobial orthodontic band cement with in situ-generated silver nanoparticles. Angle Orthod. 2015, 85, 175–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muratovska, I.; Kitagawa, H.; Hirose, N.; Kitagawa, R.; Imazato, S. Antibacterial activity and dentin bonding ability of combined use of Clearfil SE Protect and sodium hypochlorite. Dent. Mater. J. 2018, 37, 460–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nishida, M.; Imazato, S.; Takahashi, Y.; Ebisu, S.; Ishimoto, T.; Nakano, T.; Yasuda, Y.; Saito, T. The influence of the antibacterial monomer 12-methacryloyloxydodecylpyridinium bromide on the proliferation, differentiation and mineralization of odontoblast-like cells. Biomaterials 2010, 31, 1518–1532. [Google Scholar] [CrossRef] [PubMed]
- Ohmori, K.; Maeda, N.; Kohno, A. Evaluation of antibacterial activity of three dentin primers using an in vitro tooth model. Oper. Dent. 1999, 24, 279–285. [Google Scholar]
- Okeke, U.C.; Snyder, C.R.; Frukhtbeyn, S.A. Synthesis, Purification and Characterization of Polymerizable Multifunctional Quaternary Ammonium Compounds. Molecules 2019, 24, 1464. [Google Scholar] [CrossRef] [Green Version]
- Ozel, E.; Kolayli, F.; Tuna, E.B.; Er, D. In vitro antibacterial activity of various adhesive materials against oral streptococci. Biotechnol. Biotechnol. Equip. 2016, 30, 121–126. [Google Scholar] [CrossRef]
- Ozer, F.; Karakaya, S.; Unlu, N.; Erganis, O.; Kav, K.; Imazato, S. Comparison of antibacterial activity of two dentin bonding systems using agar well technique and tooth cavity model. J. Dent. 2003, 31, 111–116. [Google Scholar] [CrossRef]
- Palasuk, J.; Windsor, L.J.; Platt, J.A.; Lvov, Y.; Geraldeli, S.; Bottino, M.C. Doxycycline-loaded nanotube-modified adhesives inhibit MMP in a dose-dependent fashion. Clin. Oral Investig. 2018, 22, 1243–1252. [Google Scholar] [CrossRef]
- Pan, Y.H.; Wang, J.; Yang, Y.Q.; Nie, R.R.; Meng, X.F. Study on Preparation of Antibacterial Dental Resin Materials. J. Biomater. Tissue Eng. 2018, 8, 1580–1587. [Google Scholar] [CrossRef]
- Pedrosa, V.O.; Florio, F.M.; Turssi, C.P.; Amaral, F.L.B.; Basting, R.T.; Franca, F.M.G. Influence of pH Cycling on the Microtensile Bond Strength of Self-etching Adhesives Containing MDPB and Fluoride to Dentin and Microhardness of Enamel and Dentin Adjacent to Restorations. J. Adhes. Dent. 2012, 14, 525–534. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.L.; Carvalho, P.H.A.; Van de Sande, F.H.; Pereira, C.M.P.; Piva, E.; Lund, R.G. Self-etching dental adhesive containing a natural essential oil: Anti-biofouling performance and mechanical properties. Biofouling 2013, 29, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Peralta, S.L.; De Carvalho, P.H.A.; Ccahuana-Vasquez, R.A.; De Pereira, C.M.P.; Cury, J.A.; Piva, E.; Lund, R.G. Cytotoxicity, genotoxicity and antibiofilm activity on Streptococcus mutans of an experimental self-etching adhesive system containing natural Butia capitata oil. Int. J. Adhes. Adhes. 2017, 78, 95–101. [Google Scholar] [CrossRef]
- Pinheiro, S.L.; Soares, H.H.; Ribeiro, M.C. Microbial contamination and inhibitory effect against Streptococcus mutans from fifth-generation bonding systems. J. Appl. Biomater. Biomech. 2010, 8, 52–55. [Google Scholar] [PubMed]
- Pinto, C.F.; Berger, S.B.; Cavalli, V.; Da Cruz, S.E.B.; Goncalves, R.B.; Ambrosano, G.M.B.; Giannini, M. In situ antimicrobial activity and inhibition of secondary caries of self-etching adhesives containing an antibacterial agent and/or fluoride. Am. J. Dent. 2015, 28, 167–173. [Google Scholar] [PubMed]
- Pinto, C.F.; Leme, A.F.P.; Ambrosano, G.M.B.; Giannini, M. Effect of a Fluoride- and Bromide-containing Adhesive System on Enamel Around Composite Restorations Under High Cariogenic Challenge In Situ. J. Adhes. Dent. 2009, 11, 293–297. [Google Scholar]
- Poggio, C.; Arciola, C.R.; Cepurnykh, S.; Chiesa, M.; Scribante, A.; Selan, L.; Imbriani, M.; Visai, L. In vitro antibacterial activity of different self-etch adhesives. Int. J. Artif. Organs 2012, 35, 847–853. [Google Scholar] [CrossRef]
- Polydorou, O.; Pelz, K.; Hahn, P. Antibacterial effect of an ozone device and its comparison with two dentin-bonding systems. Eur. J. Oral Sci. 2006, 114, 349–353. [Google Scholar] [CrossRef]
- Polydorou, O.; Rogatti, P.; Bolek, R.; Wolkewitz, M.; Kummerer, K.; Hellwig, E. Elution of monomers from three different bonding systems and their antibacterial effect. Odontology 2013, 101, 170–176. [Google Scholar] [CrossRef]
- Porenczuk, A.; Grzeczkowicz, A.; Maciejewska, I.; Golas, M.; Piskorska, K.; Kolenda, A.; Gozdowski, D.; Kopec-Swoboda, E.; Granicka, L.; Olczak-Kowalczyk, D. An initial evaluation of cytotoxicity, genotoxicity and antibacterial effectiveness of a disinfection liquid containing silver nanoparticles alone and combined with a glass-ionomer cement and dentin bonding systems. Adv. Clin. Exp. Med. 2019, 28, 75–83. [Google Scholar] [CrossRef]
- Priyadarshini, B.M.; Mitali, K.; Lu, T.B.; Handral, H.K.; Dubey, N.; Fawzy, A.S. PLGA nanoparticles as chlorhexidine-delivery carrier to resin-dentin adhesive interface. Dent. Mater. 2017, 33, 830–846. [Google Scholar] [CrossRef] [PubMed]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Esmerino, L.A.; Maluf, D.F.; Zawadzki, S.F.; Michel, M.D.; Dos Santos, F.A.; Gomes, O.M.M.; Gomes, J.C. An innovative quaternary ammonium methacrylate polymer can provide improved antimicrobial properties for a dental adhesive system. J. Biomater. Sci. Polym. Ed. 2013, 24, 1443–1458. [Google Scholar] [CrossRef] [PubMed]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Kovalik, A.C.; Dos Santos, F.A.; Gomes, O.M.M.; Gomes, J.C. Effect on vascular permeability of a self-etching adhesive system containing an antimicrobial quaternary ammonium polymer QAMP into subcutaneous tissue of rats. Arch. Oral Biol. 2015, 60, 1138–1145. [Google Scholar] [CrossRef] [PubMed]
- Pupo, Y.M.; Farago, P.V.; Nadal, J.M.; Simao, L.C.; Esmerino, L.A.; Gomes, O.M.M.; Gomes, J.C. Effect of a Novel Quaternary Ammonium Methacrylate Polymer (QAMP) on Adhesion and Antibacterial Properties of Dental Adhesives. Int. J. Mol. Sci. 2014, 15, 8998–9015. [Google Scholar] [CrossRef] [Green Version]
- Pupo, Y.M.; Nadal, J.M.; Maluf, D.F.; De Lara, E.L.; Saito, R.E.; Michel, M.D.; Antunes, S.R.M.; Toledo, M.D.; Gomes, J.C.; Farago, P.V. Effect of hydroxyapatite morphology and quaternary ammonium polymer co-inclusion on bond strength, cytotoxicity, and cell morphology of self-etching adhesive. Int. J. Adhes. Adhes. 2019, 92, 7–15. [Google Scholar] [CrossRef]
- Ren, L.Y.; Pan, Y.H.; Liang, Q.; He, S.W.; Liu, Y.; Fan, Y.; Meng, X.F.; Chen, M. In Situ Synthesis of Dental Resin Matrix Containing Silver Nanoparticles. J. Nanosci. Nanotechnol. 2019, 19, 5774–5782. [Google Scholar] [CrossRef]
- Rezaeian, Z.; Beigi-Boroujeni, S.; Atai, M.; Ebrahimibagha, M.; Ozcan, M. A novel thymol-doped enamel bonding system: Physico-mechanical properties, bonding strength, and biological activity. J. Mech. Behav. Biomed. Mater. 2019, 100, 8. [Google Scholar] [CrossRef]
- Rolland, S.L.; McCabe, J.F.; Robinson, C.; Walls, A.W.G. In vitro biofilm formation on the surface of resin-based dentine adhesives. Eur. J. Oral Sci. 2006, 114, 243–249. [Google Scholar] [CrossRef]
- Rusu, L.C.; Ardelean, L.C.; Jitariu, A.A.; Miu, C.A.; Streian, C.G. An Insight into the Structural Diversity and Clinical Applicability of Polyurethanes in Biomedicine. Polymers 2020, 12, 1197. [Google Scholar] [CrossRef]
- Sabatini, C.; Mennito, A.S.; Wolf, B.J.; Pashley, D.H.; Renne, W.G. Incorporation of bactericidal poly-acrylic acid modified copper iodide particles into adhesive resins. J. Dent. 2015, 43, 546–555. [Google Scholar] [CrossRef] [Green Version]
- Schmalz, G.; Ergucu, Z.; Hiller, K.A. Effect of dentin on the antibacterial activity of dentin bonding agents. J. Endod. 2004, 30, 352–358. [Google Scholar] [CrossRef] [PubMed]
- Schmidlin, P.R.; Zehnder, M.; Gohring, T.N.; Waltimo, T.M. Glutaraldehyde in bonding systems disinfects dentin in vitro. J. Adhes. Dent. 2004, 6, 61–64. [Google Scholar] [PubMed]
- Sharma, S.; Lavender, S.; Woo, J.; Guo, L.H.; Shi, W.Y.; Kilpatrick-Liverman, L.; Gimzewski, J.K. Nanoscale characterization of effect of L-arginine on Streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology 2014, 160, 1466–1473. [Google Scholar] [CrossRef] [PubMed]
- Silva, P.A.M.; Garcia, I.M.; Nunes, J.; Visioli, F.; Leitune, V.C.B.; Melo, M.A.; Collares, F.M. Myristyltrimethylammonium Bromide (MYTAB) as a Cationic Surface Agent to Inhibit Streptococcus mutans Grown over Dental Resins: An In Vitro Study. J. Func. Biomater. 2020, 11, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, L.Y.; Ge, X.P.; Ye, Q.; Boone, K.; Xie, S.X.; Misra, A.; Tamerler, C.; Spencer, P. Modulating pH through lysine integrated dental adhesives. Dent. Mater. 2018, 34, 1652–1660. [Google Scholar] [CrossRef]
- Song, L.Y.; Ye, Q.; Ge, X.P.; Misra, A.; Tamerler, C.; Spencer, P. New silyl-functionalized BisGMA provides autonomous strengthening without leaching for dental adhesives. Acta Biomater. 2019, 83, 130–139. [Google Scholar] [CrossRef]
- Spencer, P.; Ye, Q.; Song, L.Y.; Parthasarathy, R.; Boone, K.; Misra, A.; Tamerler, C. Threats to adhesive/dentin interfacial integrity and next generation bio-enabled multifunctional adhesives. J. Biomed. Mater. Res. Part B 2019, 107, 2673–2683. [Google Scholar] [CrossRef]
- Stape, T.H.S.; Tjaderhane, L.; Tezvergil-Mutluay, A.; Yanikian, C.R.F.; Szesz, A.L.; Loguercio, A.D.; Martins, L.R.M. Dentin bond optimization using the dimethyl sulfoxide-wet bonding strategy: A 2-year in vitro study. Dent. Mater. 2016, 32, 1472–1481. [Google Scholar] [CrossRef] [Green Version]
- Stenhagen, I.S.R.; Rukke, H.V.; Dragland, I.S.; Kopperud, H.M. Effect of methacrylated chitosan incorporated in experimental composite and adhesive on mechanical properties and biofilm formation. Eur. J. Oral Sci. 2019, 127, 81–88. [Google Scholar] [CrossRef] [Green Version]
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018, 76, 283–294. [Google Scholar] [CrossRef]
- Su, M.X.; Yao, S.Y.; Gu, L.S.; Huang, Z.H.; Mai, S. Antibacterial effect and bond strength of a modified dental adhesive containing the peptide nisin. Peptides 2018, 99, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Petersen, E.J.; Watson, S.S.; Sims, C.M.; Kassman, A.; Frukhtbeyn, S.; Skrtic, D.; Ok, M.T.; Jacobs, D.S.; Reipa, V.; et al. Biophysical characterization of functionalized titania nanoparticles and their application in dental adhesives. Acta Biomater. 2017, 53, 585–597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.R.; Watson, S.S.; Allsopp, D.A.; Stanley, D.; Skrtic, D. Tuning photo-catalytic activities of TiO2 nanoparticles using dimethacrylate resins. Dent. Mater. 2016, 32, 363–372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suo, L.; Li, Z.J.; Luo, F.; Chen, J.Y.; Jia, L.L.; Wang, T.; Pei, X.B.; Wan, Q.B. Effect of dentin surface modification using carbon nanotubes on dental bonding and antibacterial ability. Dent. Mater. J. 2018, 37, 229–236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, R.; Snigdha, S.; Bhavitha, K.B.; Babu, S.; Ajith, A.; Radhakrishnan, E.K. Biofabricated silver nanoparticles incorporated polymethyl methacrylate as a dental adhesive material with antibacterial and antibiofilm activity against Streptococcus mutans. 3 Biotech 2018, 8, 10. [Google Scholar] [CrossRef]
- Thome, T.; Mayer, M.P.A.; Imazato, S.; Geraido-Martins, V.R.; Marques, M.M. In vitro analysis of inhibitory effects of the antibacterial monomer MDPB-containing restorations on the progression of secondary root caries. J. Dent. 2009, 37, 705–711. [Google Scholar] [CrossRef]
- Tiwari, S.K.; Wang, S.P.; Huang, Y.N.; Zhou, X.D.; Xu, H.H.K.; Ren, B.A.; Peng, X.; Weir, M.D.; Li, M.Y.; Cheng, L. The Antibacterial Effects of Quaternary Ammonium Salts in the Simulated Presence of Inhibitors in Root Canals: A Preliminary In-Vitro Study. Coatings 2020, 10, 181. [Google Scholar] [CrossRef] [Green Version]
- Tonini, R.; Giovarruscio, M.; Gorni, F.; Ionescu, A.; Brambilla, E.; Mikhailovna, I.M.; Luzi, A.; Pires, P.M.; Sauro, S. In Vitro Evaluation of Antibacterial Properties and Smear Layer Removal/Sealer Penetration of a Novel Silver-Citrate Root Canal Irrigant. Materials 2020, 13, 194. [Google Scholar] [CrossRef] [Green Version]
- Torres-Mendez, F.; Martinez-Castanon, G.A.; Torres-Gallegos, I.; Zavala-Alonso, N.V.; Patino-Marin, N.; Nino-Martinez, N.; Ruiz, F. Effects of silver nanoparticles on the bonding of three adhesive systems to fluorotic enamel. Dent. Mater. J. 2017, 36, 266–274. [Google Scholar] [CrossRef] [Green Version]
- Umer, D.; Yiu, C.K.Y.; Burrow, M.F.; Niu, L.N.; Tay, F.R. Effect of a novel quaternary ammonium silane on dentin protease activities. J. Dent. 2017, 58, 19–27. [Google Scholar] [CrossRef]
- Walter, R.; Duarte, W.R.; Pereira, P.N.R.; Heymann, H.O.; Swift, E.J.; Arnold, R.R. In vitro inhibition of bacterial growth using different dental adhesive systems. Oper. Dent. 2007, 32, 388–393. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.P.; Wang, H.H.; Ren, B.A.; Li, X.D.; Wang, L.; Zhou, H.; Weir, M.D.; Zhou, X.D.; Masri, R.M.; Oates, T.W.; et al. Drug resistance of oral bacteria to new antibacterial dental monomer dimethylaminohexadecyl methacrylate. Sci. Rep. 2018, 8, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, S.P.; Zhang, K.K.; Zhou, X.D.; Xu, N.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Wang, S.D.; Li, M.Y.; Li, Y.Q.; et al. Antibacterial Effect of Dental Adhesive Containing Dimethylaminododecyl Methacrylate on the Development of Streptococcus mutans Biofilm. Int. J. Mol. Sci. 2014, 15, 12791–12806. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.P.; Zhou, C.C.; Ren, B.; Li, X.D.; Weir, M.D.; Masri, R.M.; Oates, T.W.; Cheng, L.; Xu, H.K.H. Formation of persisters in Streptococcus mutans biofilms induced by antibacterial dental monomer. J. Mater. Sci. Mater. Med. 2017, 28, 10. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.Y.; Wang, B.H.; Wang, Y.H. Antibacterial orthodontic cement to combat biofilm and white spot lesions. Am. J. Orthod. Dentofac. Orthop. 2015, 148, 974–981. [Google Scholar] [CrossRef]
- Welch, K.; Cai, Y.L.; Engqvist, H.; Stromme, M. Dental adhesives with bioactive and on-demand bactericidal properties. Dent. Mater. 2010, 26, 491–499. [Google Scholar] [CrossRef]
- Wu, T.M.; Li, B.L.; Zhou, X.D.; Hu, Y.; Zhang, H.J.; Huang, Y.K.; Xu, H.H.K.; Guo, Q.; Li, M.Y.; Feng, M.Y.; et al. Evaluation of Novel Anticaries Adhesive in a Secondary Caries Animal Model. Caries Res. 2018, 52, 14–21. [Google Scholar] [CrossRef]
- Xiao, Y.H.; Ma, S.; Chen, J.H.; Chai, Z.G.; Li, F.; Wang, Y.J. Antibacterial Activity and Bonding Ability of an Adhesive Incorporating an Antibacterial Monomer DMAE-CB. J. Biomed. Mater. Res. Part B 2009, 90B, 813–817. [Google Scholar] [CrossRef]
- Xie, S.X.; Boone, K.; VanOosten, S.K.; Yuca, E.; Song, L.Y.; Ge, X.P.; Ye, Q.; Spencer, P.; Tamerler, C. Peptide Mediated Antimicrobial Dental Adhesive System. Appl. Sci. 2019, 9, 557. [Google Scholar] [CrossRef] [Green Version]
- Xie, X.J.; Wang, L.; Xing, D.; Arola, D.D.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Protein-repellent and antibacterial functions of a calcium phosphate rechargeable nanocomposite. J. Dent. 2016, 52, 15–22. [Google Scholar] [CrossRef]
- Xie, X.J.; Wang, L.; Xing, D.; Zhang, K.; Weir, M.D.; Liu, H.B.; Bai, Y.X.; Xu, H.H.K. Novel dental adhesive with triple benefits of calcium phosphate recharge, protein-repellent and antibacterial functions. Dent. Mater. 2017, 33, 553–563. [Google Scholar] [CrossRef] [PubMed]
- Xiong, J.; Shen, L.P.; Jiang, Q.S.; Kishen, A. Effect of Crosslinked Chitosan Nanoparticles on the Bonding Quality of Fiber Posts in Root Canals. J. Adhes. Dent. 2020, 22, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.J.; Li, X.J.; Zhu, W.P.; Duan, Z.H.; Mao, Y.J.; Li, X.D. Development of Novel Polymerizable Antibacterial Monomer and Its Antibacterial Effects on Dental Adhesive. Chem. J. Chin. Univ. Chin. 2019, 40, 2028–2032. [Google Scholar] [CrossRef]
- Yang, H.Y.; Li, K.; Yan, H.Y.; Liu, S.Y.; Wang, Y.K.; Huang, C. High-performance therapeutic quercetin-doped adhesive for adhesive-dentin interfaces. Sci. Rep. 2017, 7, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, S.Y.; Liu, Y.; Mao, J.; Wu, Y.B.; Deng, Y.L.; Qi, S.C.; Zhou, Y.C.; Gong, S.Q. The antibiofilm and collagen-stabilizing effects of proanthocyanidin as an auxiliary endodontic irrigant. Int. Endod. J. 2020, 53, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Yi, L.Y.; Yu, J.; Han, L.; Li, T.T.; Yang, H.Y.; Huang, C. Combination of baicalein and ethanol-wet-bonding improves dentin bonding durability. J. Dent. 2019, 90, 10. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Dong, Y.; Yu, H.H.; Lin, P.T.; Zhang, L.; Sun, X.; Liu, Y.; Xia, Y.N.; Huang, L.; Chen, J.H. Antibacterial Activity and Bonding Ability of an Orthodontic Adhesive Containing the Antibacterial Monomer 2-Methacryloxylethyl Hexadecyl Methyl Ammonium Bromide. Sci. Rep. 2017, 7, 9. [Google Scholar] [CrossRef] [Green Version]
- Yu, H.H.; Zhang, L.; Yu, F.; Li, F.; Liu, Z.Y.; Chen, J.H. Epigallocatechin-3-gallate and Epigallocatechin-3-O-(3-O-methyl)-gallate Enhance the Bonding Stability of an Etch-and-Rinse Adhesive to Dentin. Materials 2017, 10, 183. [Google Scholar] [CrossRef]
- Yue, S.C.; Wu, J.L.; Zhang, Q.; Zhang, K.; Weir, M.D.; Imazato, S.; Bai, Y.X.; Xu, H.H.K. Novel dental adhesive resin with crack self-healing, antimicrobial and remineralization properties. J. Dent. 2018, 75, 48–57. [Google Scholar] [CrossRef]
- Zhang, K.; Cheng, L.; Imazato, S.; Antonucci, J.M.; Lin, N.J.; Lin-Gibson, S.; Bai, Y.X.; Xu, H.H.K. Effects of dual antibacterial agents MDPB and nano-silver in primer on microcosm biofilm, cytotoxicity and dentine bond properties. J. Dent. 2013, 41, 464–474. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Cheng, L.; Wu, E.J.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Effect of water-ageing on dentine bond strength and anti-biofilm activity of bonding agent containing new monomer dimethylaminododecyl methacrylate. J. Dent. 2013, 41, 504–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Li, F.; Imazato, S.; Cheng, L.; Liu, H.B.; Arola, D.D.; Bai, Y.X.; Xu, H.H.K. Dual antibacterial agents of nano-silver and 12-methacryloyloxydodecylpyridinium bromide in dental adhesive to inhibit caries. J. Biomed. Mater. Res. Part B 2013, 101B, 929–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Melo, M.A.S.; Cheng, L.; Weir, M.D.; Bai, Y.; Xu, H.H.K. Effect of quaternary ammonium and silver nanoparticle-containing adhesives on dentin bond strength and dental plaque microcosm biofilms. Dent. Mater. 2012, 28, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, K.; Wang, S.; Zhou, X.; Xu, H.H.K.; Weir, M.D.; Ge, Y.; Li, M.; Wang, S.; Li, Y.; Xu, X.; et al. Effect of Antibacterial Dental Adhesive on Multispecies Biofilms Formation. J. Dent. Res. 2015, 94, 622–629. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, N.; Chen, C.; Weir, M.D.; Bai, Y.X.; Xu, H.H.K. Antibacterial and protein-repellent orthodontic cement to combat biofilms and white spot lesions. J. Dent. 2015, 43, 1529–1538. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Melo, M.A.S.; Bai, Y.X.; Xu, H.H.K. Novel protein-repellent dental adhesive containing 2-methacryloyloxyethyl phosphorylcholine. J. Dent. 2014, 42, 1284–1291. [Google Scholar] [CrossRef] [Green Version]
- Zhang, N.; Weir, M.D.; Romberg, E.; Bai, Y.X.; Xu, H.H.K. Development of novel dental adhesive with double benefits of protein-repellent and antibacterial capabilities. Dent. Mater. 2015, 31, 845–854. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, K.; Weir, M.D.; Xu, D.J.; Reynolds, M.A.; Bai, Y.X.; Xu, H.H.K. Effects of water-aging for 6 months on the durability of a novel antimicrobial and protein-repellent dental bonding agent. Int. J. Oral Sci. 2018, 10, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Zhang, W.; Luo, X.J.; Niu, L.N.; Liu, S.Y.; Zhu, W.C.; Epasinghe, J.; Chen, L.; Li, G.H.; Huang, C.; Mao, J.; et al. One-pot synthesis of antibacterial monomers with dual biocidal modes. J. Dent. 2014, 42, 1078–1095. [Google Scholar] [CrossRef]
- Zhao, M.D.; Qu, Y.; Liu, J.; Mai, S.; Gu, L.S. A universal adhesive incorporating antimicrobial peptide nisin: Effects on Streptococcus mutans and saliva-derived multispecies biofilms. Odontology 2020, 108, 376–385. [Google Scholar] [CrossRef]
- Zhao, Z.L.; Lian, Y.Q. Synthesis of New Antibacterial Acrylic Monomer and Its Application in Dental Restoration Resin-based. Chem. J. Chin. Univ. Chin. 2013, 34, 708–713. [Google Scholar] [CrossRef]
- Zhou, H.; Li, F.; Weir, M.D.; Xu, H.H.K. Dental plaque microcosm response to bonding agents containing quaternary ammonium methacrylates with different chain lengths and charge densities. J. Dent. 2013, 41, 1122–1131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Liu, H.B.; Weir, M.D.; Reynolds, M.A.; Zhang, K.; Xu, H.H.K. Three-dimensional biofilm properties on dental bonding agent with varying quaternary ammonium charge densities. J. Dent. 2016, 53, 73–81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, H.; Weir, M.D.; Antonucci, J.M.; Schumacher, G.E.; Zhou, X.D.; Xu, H.H.K. Evaluation of three-dimensional biofilms on antibacterial bonding agents containing novel quaternary ammonium methacrylates. Int. J. Oral Sci. 2014, 6, 77–86. [Google Scholar] [CrossRef] [Green Version]
- Clarivate Analytics. InCites Indicators Handbook; Clarivate Analytics: Philadelphia, PA, USA, 2018. [Google Scholar]
- Available online: https://support.clarivate.com/ScientificandAcademicResearch/s/article/Web-of-Science-Core-Collection-List-of-field-tags-in-output?language=en_US (accessed on 27 November 2020).
- Gutmann, J.L. The evolution of America’s scientific advancements in dentistry in the past 150 years. J. Am. Dent. Assoc. 2009, 140 (Suppl. 1), 8s–15s. [Google Scholar] [CrossRef]
- Imazato, S.; Chen, J.-h.; Ma, S.; Izutani, N.; Li, F. Antibacterial resin monomers based on quaternary ammonium and their benefits in restorative dentistry. Jpn. Dent. Sci. Rev. 2012, 48, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Imazato, S.; McCabe, J.F. Influence of Incorporation of Antibacterial Monomer on Curing Behavior of a Dental Composite. J. Dent. Res. 1994, 73, 1641–1645. [Google Scholar] [CrossRef] [Green Version]
- Moazami, F.; Sahebi, S.; Ahzan, S. Tooth Discoloration Induced by Imidazolium Based Silver Nanoparticles as an Intracanal Irrigant. J. Dent. 2018, 19, 280–286. [Google Scholar]
- Okamura, K. Interdisciplinarity revisited: Evidence for research impact and dynamism. Palgrave Commun. 2019, 5, 141. [Google Scholar] [CrossRef]








| Rank | Country | TP | TC | CI | Rank | Organizations | Country | TP | TC | CI |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | USA | 99 | 2480 | 25.05 | 1 | The University of Maryland | USA | 53 | 1713 | 32.32 |
| 2 | China | 91 | 2473 | 27.18 | 2 | Sichuan University | China | 32 | 1128 | 35.25 |
| 3 | Brazil | 59 | 861 | 14.59 | 3 | Capital Medical University | China | 25 | 877 | 35.08 |
| 4 | Japan | 22 | 917 | 41.68 | 4 | Fourth Military Medical University | China | 24 | 984 | 41.00 |
| 5 | Turkey | 13 | 195 | 15.00 | 5 | University of Maryland, Baltimore County | USA | 21 | 932 | 44.38 |
| 6 | Canada | 8 | 44 | 5.50 | 6 | Osaka University | Japan | 16 | 810 | 50.63 |
| 7 | Italy | 8 | 137 | 17.13 | 7 | Universidade Estadual De Campinas | Brazil | 12 | 93 | 7.75 |
| 8 | Germany | 6 | 131 | 21.83 | 8 | Federal University of Rio Grande do Sul | Brazil | 11 | 71 | 6.45 |
| 9 | South Korea | 6 | 199 | 33.17 | 9 | Universidade Estadual de Ponta Grossa | Brazil | 9 | 72 | 8.00 |
| 10 | Spain | 6 | 281 | 46.83 | 10 | National Institute of Standards and Technology | USA | 7 | 164 | 23.43 |
| Source | TP | TC | CI | Country | Publisher | IF | Q |
|---|---|---|---|---|---|---|---|
| Dental Materials | 40 | 1468 | 36.70 | The Netherlands | Elsevier | 4.5 | 1 |
| Journal of Dentistry | 29 | 747 | 25.76 | The Netherlands | Elsevier | 3.24 | 1 |
| Acta Biomaterialia | 10 | 312 | 31.20 | The Netherlands | Elsevier | 7.24 | 1 |
| Journal of Dental Research | 10 | 594 | 59.40 | USA | Sage | 4.91 | 1 |
| Journal of Adhesive Dentistry | 9 | 119 | 13.22 | USA | Quintessence Publishing | 2.38 | 2 |
| Journal of Biomedical Materials Research Part B-Applied Biomaterials | 9 | 246 | 27.33 | USA | Wiley | 2.83 | 3 |
| International Journal of Adhesion and Adhesives | 8 | 21 | 2.63 | The Netherlands | Elsevier | 2.67 | 2 |
| Journal of The Mechanical Behavior of Biomedical Materials | 6 | 13 | 2.17 | The Netherlands | Elsevier | 3.37 | 2 |
| Operative Dentistry | 6 | 176 | 29.33 | USA | Operative Dentistry | 2.21 | 2 |
| Dental Materials Journal | 5 | 14 | 2.80 | Japan | Japanese Society for Dental Materials and Devices | 1.36 | 3 |
| Title | Author | Source | TC | C/Y | Year |
|---|---|---|---|---|---|
| Antibacterial Activity of Dental Composites Containing Quaternary Ammonium Polyethylenimine Nanoparticles Against Streptococcus Mutans | Beyth et al. [55] | Biomaterials | 300 | 21.43 | 2006 |
| Antibacterial Activity and Bonding Characteristics of An Adhesive Resin Containing Antibacterial Monomer MDPB | Imazato et al. [137] | Dental Materials | 181 | 10.65 | 2003 |
| Incorporation of Antibacterial Monomer MDPB into Dentin Primer | Imazato et al. [138] | Journal of Dental Research | 148 | 11.38 | 1997 |
| Experimental Antimicrobial Orthodontic Adhesives Using Nanofillers And Silver Nanoparticles | Ahn et al. [36] | Dental Materials | 146 | 13.27 | 2009 |
| In Vitro Antibacterial Effects of The Dentin Primer of Clearfil Protect Bond | Imazato et al. [139] | Dental Materials | 138 | 9.86 | 2006 |
| Effects of a Dental Adhesive Incorporating Antibacterial Monomer on The Growth, Adherence and Membrane Integrity of Streptococcus Mutans | Li et al. [160] | Journal of Dentistry | 133 | 12.09 | 2009 |
| Anti-Biofilm Dentin Primer with Quaternary Ammonium and Silver Nanoparticles | Cheng et al. [72] | Journal of Dental Research | 116 | 14.50 | 2012 |
| An In Vivo Evaluation of Bonding Ability of Comprehensive Antibacterial Adhesive System Incorporating MDPB | Imazato et al. [141] | Dental Materials | 106 | 8.15 | 2007 |
| Effect of Quaternary Ammonium and Silver Nanoparticle-Containing Adhesives on Dentin Bond Strength and Dental Plaque Microcosm Biofilms | Zhang et al. [269] | Dental Materials | 98 | 12.25 | 2012 |
| Novel Dental Adhesives Containing Nanoparticles of Silver and Amorphous Calcium Phosphate | Melo et al. [191] | Dental Materials | 95 | 101 | 2013 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khan, A.S.; Ur Rehman, S.; AlMaimouni, Y.K.; Ahmad, S.; Khan, M.; Ashiq, M. Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020. Polymers 2020, 12, 2848. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122848
Khan AS, Ur Rehman S, AlMaimouni YK, Ahmad S, Khan M, Ashiq M. Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020. Polymers. 2020; 12(12):2848. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122848
Chicago/Turabian StyleKhan, Abdul Samad, Shafiq Ur Rehman, Yara Khalid AlMaimouni, Shakil Ahmad, Maria Khan, and Murtaza Ashiq. 2020. "Bibliometric Analysis of Literature Published on Antibacterial Dental Adhesive from 1996–2020" Polymers 12, no. 12: 2848. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12122848