Evaluation of Carbon Dioxide-Based Urethane Acrylate Composites for Sealers of Root Canal Obturation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of Urethane-acrylate (UA)
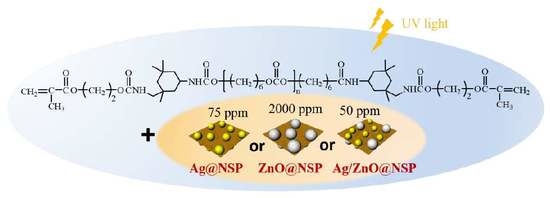
2.2. Preparation of Antibacterial Sealer Based on UA
2.3. Curing Condition and Depth Test
2.4. Biocompatibility Test
2.5. Antibacterial Test
3. Results and Discussion
3.1. Synthesis of Urethan-acrylate (UA)
3.2. Preparation and Thermal Properties of UA Composites
3.3. Curing Conditions and Depths for UA Composites
3.4. Biocompatibility Analysis
3.5. Antibacterial Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Yang, H.; Tian, C.; Li, G.; Yang, L.; Han, X.; Wang, Y. A cone-beam computed tomography study of the root canal morphology of mandibular first premolars and the location of root canal orifices and apical foramina in a chinese subpopulation. J. Endod. 2013, 39, 435–438. [Google Scholar] [CrossRef] [PubMed]
- Carrotte, P. Endodontics: Part 3 Treatment of endodontic emergencies. Br. Dent. J. 2004, 197, 299–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hsieh, K.-H.; Liao, K.-H.; Lai, E.H.-H.; Lee, B.-S.; Lee, C.-Y.; Lin, C.-P. A novel polyurethane-based root canal-obturation material and urethane acrylate-based root canal sealer—Part I: Synthesis and evaluation of mechanical and thermal properties. J. Endod. 2008, 34, 303–305. [Google Scholar] [CrossRef]
- Carrotte, P. Endodontics: Part 5 Basic instruments and materials for root canal treatment. Br. Dent. J. 2004, 197, 455–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Al-Hiyasat, A.S.; Alfirjani, S.A. The effect of obturation techniques on the push-out bond strength of a premixed bioceramic root canal sealer. J. Dent. 2019, 89, 103169. [Google Scholar] [CrossRef] [PubMed]
- Schäfer, E.; Zandbiglari, T. Solubility of root-canal sealers in water and artificial saliva. Int. Endod. J. 2003, 36, 660–669. [Google Scholar] [CrossRef] [PubMed]
- Wennber, A.; Niom, D.Ø. Adhesion of root canal sealers to bovine dentine and gutta-percha. Int. Endod. J. 1990, 23, 13–19. [Google Scholar] [CrossRef]
- Washio, A.; Morotomi, T.; Yoshii, S.; Kitamura, C. Bioactive glass-based endodontic sealer as a promising root canal filling material without semisolid core materials. Materials 2019, 12, 3967. [Google Scholar] [CrossRef] [Green Version]
- Teoh, Y.-Y.; Athanassiadis, B.; Walsh, L.J. Sealing ability of alkaline endodontic cements versus resin cements. Materials 2017, 10, 1228. [Google Scholar] [CrossRef] [Green Version]
- Tyagi, S.; Mishra, P.; Tyagi, P. Evolution of root canal sealers: An insight story. Eur. J. Gen. Dent. 2013, 2, 199–218. [Google Scholar] [CrossRef]
- De-Deus, G.; Canabarro, A.; Alves, G.G.; Marins, J.R.; Linhares, A.B.R.; Granjeiro, J.M. Cytocompatibility of the ready-to-use bioceramic putty repair cement iRoot BP Plus with primary human osteoblasts. Int. Endod. J. 2012, 45, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Pawar, A.M.; Pawar, S.; Kfir, A.; Pawar, M.; Kokate, S. Push-out bond strength of root fillings made with C-Point and BC sealer versus gutta-percha and AH Plus after the instrumentation of oval canals with the self-adjusting file versus waveone. Int. Endod. J. 2016, 49, 374–381. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Lai, E.H.-H.; Liao, K.-H.; Lee, C.-Y.; Hsieh, K.-H.; Lin, C.-P. A novel polyurethane-based root canal-obturation material and urethane-acrylate-based root canal sealer-Part 2: Evaluation of Push-out Bond Strengths. J. Endod. 2008, 34, 594–598. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.-S.; Wang, C.-Y.; Fang, Y.-Y.; Hsieh, K.-H.; Lin, C.S. A novel urethane acrylate-based root canal sealer with improved degree of conversion, cytotoxicity, bond strengths, solubility, and dimensional stability. J. Endod. 2011, 37, 246–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.-Y.; Huang, C.-K.; Lin, S.-P.; Han, J.-L.; Hsieh, K.-H.; Lin, C.-P. Low-shrinkage visible-light-curable urethane-modified epoxy acrylate/SiO2 composites as dental restorative materials. Compos. Sci. Technol. 2008, 68, 2811–2817. [Google Scholar] [CrossRef]
- Tay, F.R.; Loushine, R.J.; Weller, R.N.; Kimbrough, W.F.; Pashley, D.H.; Mak, Y.-F.; Shirley Lai, C.-N.; Raina, R.; Williams, M.C. Ultrastructural evaluation of the apical seal in roots filled with a polycaprolactone-based root canal filling material. J. Endod. 2005, 31, 514–519. [Google Scholar] [CrossRef]
- Delebecq, E.; Pascault, J.-P.; Boutevin, B.; Ganachaud, F. On the versatility of urethane/urea bonds: Reversibility, blocked isocyanate, and non-isocyanate polyurethane. Chem. Rev. 2013, 113, 80–118. [Google Scholar] [CrossRef]
- Lin, J.-J.; Chu, C.-C.; Chiang, M.-L.; Tsai, W.-C. First isolation of individual silicate platelets from clay exfoliation and their unique self-assembly into fibrous arrays. J. Phys. Chem. B 2006, 110, 18115–18120. [Google Scholar] [CrossRef]
- Chu, C.-C.; Chiang, M.-L.; Tsai, C.-M.; Lin, J.-J. Exfoliation of montmorillonite clay by mannich polyamines with multiple quaternary salts. Macromolecules 2005, 38, 6240–6243. [Google Scholar] [CrossRef]
- Dong, R.-X.; Chou, C.-C.; Lin, J.-J. Synthesis of immobilized silver nanoparticles on ionic silicate clay and observed low-temperature melting. J. Mater. Chem. 2009, 19, 2184–2188. [Google Scholar] [CrossRef]
- Su, H.-L.; Lin, S.-H.; Wei, J.-C.; Pao, I.C.; Chiao, S.-H.; Huang, C.-C.; Lin, S.-Z.; Lin, J.-J. Novel nanohybrids of silver particles on clay platelets for inhibiting silver-resistant bacteria. PLoS ONE 2011, 6, e21125. [Google Scholar] [CrossRef] [PubMed]
- Chu, C.-Y.; Peng, F.-C.; Chiu, Y.-F.; Lee, H.-C.; Chen, C.-W.; Wei, J.-C.; Lin, J.-J. Nanohybrids of silver particles immobilized on silicate platelet for infected wound healing. PLoS ONE 2012, 7, e38360. [Google Scholar] [CrossRef]
- Janaki, S.; Sailatha, E.; Gunasekaran, S. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 144, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Wahid, F.; Yin, J.-J.; Xue, D.-D.; Xue, H.; Lu, Y.-S.; Zhong, C.; Chu, L.-Q. Synthesis and characterization of antibacterial carboxymethyl Chitosan/ZnO nanocomposite hydrogels. Int. J. Biol. Macromol. 2016, 88, 273–279. [Google Scholar] [CrossRef] [PubMed]
- Sirelkhatim, A.; Mahmud, S.; Seeni, A.; Kaus, N.H.M.; Ann, L.C.; Bakhori, S.K.M.; Hasan, H.; Mohamad, D. Review on zinc oxide nanoparticles: Antibacterial activity and toxicity mechanism. Nano-Micro Lett. 2015, 7, 219–242. [Google Scholar] [CrossRef] [Green Version]
- Cao, N.; Pegoraro, M.; Severini, F.; Di Landro, L.; Zoia, G.; Greco, A. Crosslinked polycarbonate polyurethanes: Preparation and physical properties. Polymer 1992, 33, 1384–1390. [Google Scholar] [CrossRef]
- Naik, P.U.; Refes, K.; Sadaka, F.; Brachais, C.-H.; Boni, G.; Couvercelle, J.-P.; Picquet, M.; Plasseraud, L. Organo-catalyzed synthesis of aliphatic polycarbonates in solvent-free conditions. Polym. Chem. 2012, 3, 1475–1480. [Google Scholar] [CrossRef]
- Khan, I.; Smith, N.; Jones, E.; Finch, D.; Cameron, R. Analysis and evaluation of a biomedical polycarbonate urethane tested in an in vitro study and an ovine arthroplasty model. Part II: In vivo investigation. Biomaterials 2005, 26, 633–643. [Google Scholar] [CrossRef]
- Zhu, W.; Wang, Y.; Sun, S.; Zhang, Q.; Li, X.; Shen, Z. Facile synthesis and characterization of biodegradable antimicrobial poly(ester-carbonate). J. Mater. Chem. 2012, 22, 11785–11791. [Google Scholar] [CrossRef]
- Rueggeberg, F.A. Determination of resin cure using infrared analysis without an internal standard. Dent. Mater. 1994, 10, 282–286. [Google Scholar] [CrossRef]
- Almeida, J.F.A.; Gomes, B.P.F.A.; Ferraz, C.C.R.; Souza-Filho, F.J.; Zaia, A.A. Filling of artificial lateral canals and microleakage and flow of five endodontic sealers. Int. Endod. J. 2007, 40, 692–699. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.-m.; Shen, Y.; Zheng, W.; Li, L.; Zheng, Y.-f.; Haapasalo, M. Physical properties of 5 root canal sealers. J. Endod. 2013, 39, 1281–1286. [Google Scholar] [CrossRef] [PubMed]
- Goracci, C.; Cadenaro, M.; Fontanive, L.; Giangrosso, G.; Juloski, J.; Vichi, A.; Ferrari, M. Polymerization efficiency and flexural strength of low-stress restorative composites. Dent. Mater. 2014, 30, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Schmalz, G. Concepts in biocompatibility testing of dental restorative materials. Clin. Oral Investig. 1998, 1, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Shin, T.P.; Yao, X.; Huenergardt, R.; Walker, M.P.; Wang, Y. Morphological and chemical characterization of bonding hydrophobic adhesive to dentin using ethanol wet bonding technique. Dent. Mater. 2009, 25, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, C.-H.; Lin, Y.-R.; Yeh, S.-C.; Huang, Y.-C.; Sun, K.-H.; Shih, Y.-F.; Su, W.-C.; Dai, C.-A.; Dai, S.A.; Jeng, R.-J. A facile synthetic route to ether diols derived from 1,1-cyclopentylenylbisphenol for robust cardo-type polyurethanes. Macromolecules 2019, 52, 959–967. [Google Scholar] [CrossRef]
- Wu, C.-H.; Chen, L.-Y.; Jeng, R.-J.; Dai, S.A. 100% Atom-economy efficiency of recycling polycarbonate into versatile intermediates. ACS Sustain. Chem. Eng. 2018, 6, 8964–8975. [Google Scholar] [CrossRef]
- Nabinejad, O.; Sujan, D.; Rahman, M.E.; Davies, I.J. Determination of filler content for natural filler polymer composite by thermogravimetric analysis. J. Therm. Anal. Calorim. 2015, 122, 227–233. [Google Scholar] [CrossRef]
- Hajipour, M.J.; Fromm, K.M.; Akbar Ashkarran, A.; Jimenez de Aberasturi, D.; Larramendi, I.R.d.; Rojo, T.; Serpooshan, V.; Parak, W.J.; Mahmoudi, M. Antibacterial properties of nanoparticles. Trends Biotechnol. 2012, 30, 499–511. [Google Scholar] [CrossRef] [Green Version]
- Mustapha, S.; Ndamitso, M.M.; Abdulkareem, A.S.; Tijani, J.O.; Shuaib, D.T.; Ajala, A.O.; Mohammed, A.K. Application of TiO2 and ZnO nanoparticles immobilized on clay in wastewater treatment: A review. Appl. Water Sci. 2020, 10, 1–36. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Qin, Y.; Wang, X.; Zhao, X.; Wang, F. Plasticizing while toughening and reinforcing poly(propylene carbonate) using low molecular weight urethane: Role of hydrogen-bonding interaction. Polymer 2011, 52, 4873–4880. [Google Scholar] [CrossRef]
- Ma, X.; Yu, J.; Wang, N. Compatibility characterization of poly(lactic acid)/poly(propylene carbonate) blends. J. Polym. Sci. Part B Polym. Phys. 2006, 44, 94–101. [Google Scholar] [CrossRef]
- Xu, H.; Qiu, F.; Wang, Y.; Wu, W.; Yang, D.; Guo, Q. UV-curable waterborne polyurethane-acrylate: Preparation, characterization and properties. Prog. Org. Coat. 2012, 73, 47–53. [Google Scholar] [CrossRef]
- Balan, L.; Schneider, R.; Lougnot, D.J. A new and convenient route to polyacrylate/silver nanocomposites by light-induced cross-linking polymerization. Prog. Org. Coat. 2008, 62, 351–357. [Google Scholar] [CrossRef]
- Gopikrishna, V.; Suresh Chandra, B. Grossman’s Endodontic Practice, 13th ed.; Wolters Kluwer Health: Philadelphia, PA, USA, 2014. [Google Scholar]
- Faccioni, F.; Franceschetti, P.; Cerpelloni, M.; Fracasso, M. In vivo study on metal ion release fixed orthodontic appliances and DNA damage in oral mucosa cells. Am. J. Orthod. Dentofac. Orthop. 2003, 124, 687–693. [Google Scholar] [CrossRef]
- Eldeniz, A.; Mustafa, K.; Ørstavik, D.; Dahl, J. Cytotoxicity of new resin-, calcium hydroxide- and silicone-based root canal sealers on fibroblasts derived from human gingiva and L929 cell lines. Int. Endod. J. 2007, 40, 329–337. [Google Scholar] [CrossRef]
- Al-Hiyasat, A.; Tayyar, M.; Darmani, H. Cytotoxicity evaluation of various resin based root canal sealers. Int. Endod. J. 2010, 43, 148–153. [Google Scholar] [CrossRef]
- Mathur, A.B.; Collier, T.O.; Kao, W.J.; Wiggins, M.; Schubert, M.A.; Hiltner, A.; Anderson, J.M. In vivo biocompatibility and biostability of modified polyurethanes. J. Biomed. Mater. Res. 1997, 36, 246–257. [Google Scholar] [CrossRef]
- Schubert, M.A.; Wiggins, M.J.; Schaefer, M.P.; Hiltner, A.; Anderson, J.M. Oxidative biodegradation mechanisms of biaxially strained poly(etherurethane urea) elastomers. J. Biomed. Mater. Res. 1995, 29, 337–347. [Google Scholar] [CrossRef]
- Wei, J.-C.; Yen, Y.-T.; Su, H.-L.; Lin, J.-J. Inhibition of bacterial growth by the exfoliated clays and observation of physical capturing mechanism. J. Phys. Chem. C 2011, 115, 18770–18775. [Google Scholar] [CrossRef]
















| Entry | UA Formulation | Photoinitiator (phr 1) | Tensile Strength (MPa) | Young’s Modulus (MPa) | DC 2 (%) |
|---|---|---|---|---|---|
| 1 | UAT65/TPGDA (w/w = 70/30) | 1 | 51.71 ± 3.03 | 362.81 ± 37.10 | 46.27 ± 3.07 |
| 2 | UAT65/TPGDA (w/w = 70/30) | 3 | 56.15 ± 3.26 | 361.88 ± 32.38 | 64.91 ± 1.06 |
| 3 | UAT65/TPGDA (w/w = 70/30) | 6 | 45.96 ± 2.22 | 33.49 ± 1.65 | 67.58 ± 2.27 |
| 4 | UAT65/TPGDA (w/w = 70/30) | 9 | 36.78 ± 1.65 | 1.92 ± 0.42 | 70.35 ± 1.76 |
| 5 | UAC50/TPGDA (w/w = 70/30) | 1 | 46.30 ± 3.18 | 1072.25 ± 46.54 | 57.89 ± 0.41 |
| 6 | UAC50/TPGDA (w/w = 70/30) | 3 | 60.54 ± 4.72 | 1042.02 ± 39.62 | 72.44 ± 1.57 |
| 7 | UAC50/TPGDA (w/w = 70/30) | 6 | 61.09 ± 1.15 | 14.01 ± 2.65 | 79.03 ± 2.57 |
| 8 | UAC50/TPGDA (w/w = 70/30) | 9 | 59.04 ± 2.75 | 8.61 ± 0.41 | 80.08 ± 2.79 |
| Entry | Type of Composites (1) | Antibacterial Activity (2) |
|---|---|---|
| 1 | Ag@NSP-50 | ╳ |
| 2 | Ag@NSP-75 | ○ |
| 3 | Ag@NSP-100 | ○ |
| 4 | ZnO@NSP-100 | ╳ |
| 5 | ZnO@NSP-200 | ╳ |
| 6 | ZnO@NSP-300 | ╳ |
| 7 | ZnO@NSP-500 | ╳ |
| 8 | ZnO@NSP-1000 | ╳ |
| 9 | ZnO@NSP-2000 | ○ |
| 10 | ZnO@NSP-3000 | ○ |
| 11 | Ag/ZnO@NSP-10 | ╳ |
| 12 | Ag/ZnO@NSP-25 | ╳ |
| 13 | Ag/ZnO@NSP-50 | ○ |
| 14 | Ag/ZnO@NSP-75 | ○ |
| 15 | Ag/ZnO@NSP-100 | ○ |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, H.-H.; Tseng, Y.-T.; Huang, S.-W.; Kuo, Y.-F.; Yeh, C.-L.; Wu, C.-H.; Huang, Y.-C.; Jeng, R.-J.; Lin, J.-J.; Lin, C.-P. Evaluation of Carbon Dioxide-Based Urethane Acrylate Composites for Sealers of Root Canal Obturation. Polymers 2020, 12, 482. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020482
Chang H-H, Tseng Y-T, Huang S-W, Kuo Y-F, Yeh C-L, Wu C-H, Huang Y-C, Jeng R-J, Lin J-J, Lin C-P. Evaluation of Carbon Dioxide-Based Urethane Acrylate Composites for Sealers of Root Canal Obturation. Polymers. 2020; 12(2):482. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020482
Chicago/Turabian StyleChang, Hao-Hueng, Yi-Ting Tseng, Sheng-Wun Huang, Yi-Fang Kuo, Chun-Liang Yeh, Chien-Hsin Wu, Ying-Chi Huang, Ru-Jong Jeng, Jiang-Jen Lin, and Chun-Pin Lin. 2020. "Evaluation of Carbon Dioxide-Based Urethane Acrylate Composites for Sealers of Root Canal Obturation" Polymers 12, no. 2: 482. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12020482