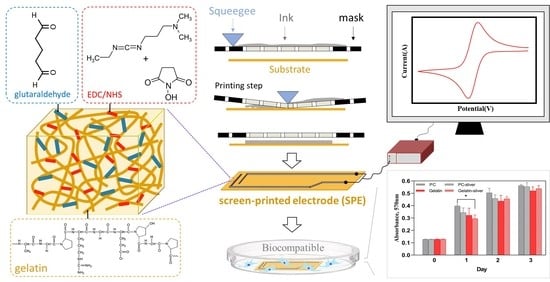
A Facile Fabrication of Biodegradable and Biocompatible Cross-Linked Gelatin as Screen Printing Substrates
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
2.2. Preparation of Gelatin Film
2.3. Swelling Test of Gelatin Film
2.4. Mechanical Strength Test of Gelatin Film
2.5. Fabrication of Screen-Printed Electrode (SPE) on Gelatin
2.6. Adhesion Test of the SPE
2.7. Cyclic Voltammetry (CV) Measurement
2.8. Cell Biocompatibility Assay
2.9. Statistics
3. Results and Discussion
3.1. Characterization of Gelatin Films
3.1.1. Morphology of Gelatin Film
3.1.2. Swelling Ratio of Gelatin Films
3.1.3. Mechanical Properties of Gelatin Films
3.2. Gelatin Film as Screen-Printing Electrode Substrate
3.2.1. Adhesion Test of SPE
3.2.2. Electrochemical Characterization of SPE
3.2.3. Cell Viability Assay
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Desmond, D.; Lane, B.; Alderman, J.; Glennon, J.; Diamond, D.; Arrigan, D. Evaluation of miniaturised solid state reference electrodes on a silicon based component. Sens. Actuators B Chem. 1997, 44, 389–396. [Google Scholar] [CrossRef]
- Naqvi, S.T.R.; Rasheed, T.; Ashiq, M.N.; ul Haq, M.N.; Majeed, S.; Fatima, B.; Nawaz, R.; Hussain, D.; Shafi, S. Fabrication of iron modified screen printed carbon electrode for sensing of amino acids. Polyhedron 2020, 180, 114426. [Google Scholar] [CrossRef]
- Roshanghias, A.; Rodrigues, A.D.; Holzmann, D. Thermosonic fine-pitch flipchip bonding of silicon chips on screen printed paper and PET substrates. Microelectron. Eng. 2020, 228, 111330. [Google Scholar] [CrossRef]
- Adly, N.; Weidlich, S.; Seyock, S.; Brings, F.; Yakushenko, A.; Offenhäusser, A.; Wolfrum, B. Printed microelectrode arrays on soft materials: From PDMS to hydrogels. npj Flex. Electron. 2018, 2, 15. [Google Scholar] [CrossRef]
- Polikov, V.S.; Tresco, P.A.; Reichert, W.M. Response of brain tissue to chronically implanted neural electrodes. Neurosci Methods 2005, 148, 1–18. [Google Scholar] [CrossRef]
- Seo, J.-M.; Kim, S.J.; Chung, H.; Kim, E.T.; Yu, H.G.; Yu, Y.S. Biocompatibility of polyimide microelectrode array for retinal stimulation. Mater. Sci. Eng. 2004, 24, 185–189. [Google Scholar] [CrossRef]
- Rodger, D.C.; Fong, A.J.; Li, W.; Ameri, H.; Ahuja, A.K.; Gutierrez, C.; Lavrov, I.; Zhong, H.; Menon, P.R.; Meng, E. Flexible parylene-based multielectrode array technology for high-density neural stimulation and recording. Sens. Actuators B Chem. 2008, 132, 449–460. [Google Scholar] [CrossRef] [Green Version]
- Meacham, K.W.; Giuly, R.J.; Guo, L.; Hochman, S.; DeWeerth, S.P. A lithographically-patterned, elastic multi-electrode array for surface stimulation of the spinal cord. Biomed. Microdevices 2008, 10, 259–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jo, B.-H.; Van Lerberghe, L.M.; Motsegood, K.M.; Beebe, D.J. Three-dimensional micro-channel fabrication in polydimethylsiloxane (PDMS) elastomer. J. Microelectromech. Syst. 2000, 9, 76–81. [Google Scholar] [CrossRef]
- Mata, A.; Boehm, C.; Fleischman, A.J.; Muschler, G.; Roy, S. Growth of connective tissue progenitor cells on microtextured polydimethylsiloxane surfaces. J. Biomed. Mater. Res. Off. J. Soc. Biomater. Jpn. Soc. Biomater. Aust. Soc. Biomater. Korean Soc. Biomater. 2002, 62, 499–506. [Google Scholar] [CrossRef] [PubMed]
- Larmagnac, A.; Eggenberger, S.; Janossy, H.; Vörös, J. Stretchable electronics based on Ag-PDMS composites. Sci. Rep. 2014, 4, 7254. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trantidou, T.; Elani, Y.; Parsons, E.; Ces, O. Hydrophilic surface modification of PDMS for droplet microfluidics using a simple, quick, and robust method via PVA deposition. Microsyst. Nanoeng. 2017, 3, 16091. [Google Scholar] [CrossRef] [PubMed]
- Tu, Q.; Wang, J.-C.; Zhang, Y.; Liu, R.; Liu, W.; Ren, L.; Shen, S.; Xu, J.; Zhao, L.; Wang, J. Surface modification of poly (dimethylsiloxane) and its applications in microfluidics-based biological analysis. Rev. Anal. Chem. 2012, 31, 177–192. [Google Scholar] [CrossRef]
- Kovach, K.M.; Capadona, J.R.; Gupta, A.S.; Potkay, J.A. The effects of PEG-based surface modification of PDMS microchannels on long-term hemocompatibility. J. Biomed. Mater. Res. Part A 2014, 102, 4195–4205. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; He, J.; Ren, X.; Cai, W.-S.; Fang, Y.-C.; Feng, X.-Z. Development of functional biointerfaces by surface modification of polydimethylsiloxane with bioactive chlorogenic acid. Colloids Surf. B Biointerfaces 2014, 116, 700–706. [Google Scholar] [CrossRef] [PubMed]
- Nawroth, J.C.; Scudder, L.L.; Halvorson, R.T.; Tresback, J.; Ferrier, J.P., Jr.; Sheehy, S.P.; Cho, A.; Kannan, S.; Sunyovszki, I.; Goss, J.A. Automated fabrication of photopatterned gelatin hydrogels for organ-on-chips applications. Biofabrication 2018, 10, 025004. [Google Scholar] [CrossRef]
- Cunningham, J.J.; Nikolovski, J.; Linderman, J.J.; Mooney, D.J. Quantification of fibronectin adsorption to silicone-rubber cell culture substrates. Biotechniques 2002, 32, 876–887. [Google Scholar] [CrossRef]
- Su, K.; Wang, C. Recent advances in the use of gelatin in biomedical research. Biotechnol. Lett. 2015, 37, 2139–2145. [Google Scholar] [CrossRef]
- Aldana, A.A.; Abraham, G.A. Current advances in electrospun gelatin-based scaffolds for tissue engineering applications. Int. J. Pharm. 2017, 523, 441–453. [Google Scholar] [CrossRef] [Green Version]
- Yue, K.; Trujillo-de Santiago, G.; Alvarez, M.M.; Tamayol, A.; Annabi, N.; Khademhosseini, A. Synthesis, properties, and biomedical applications of gelatin methacryloyl (GelMA) hydrogels. Biomaterials 2015, 73, 254–271. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Ao, Q.; Tian, X.; Fan, J.; Tong, H.; Hou, W.; Bai, S. Gelatin-based hydrogels for organ 3D bioprinting. Polymers 2017, 9, 401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaipan, P.; Nguyen, A.; Narayan, R.J. Gelatin-based hydrogels for biomedical applications. MRS Commun. 2017, 7, 416–426. [Google Scholar] [CrossRef]
- Campiglio, C.E.; Contessi Negrini, N.; Farè, S.; Draghi, L.J.M. Cross-linking strategies for electrospun gelatin scaffolds. Materials 2019, 12, 2476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bello, A.B.; Kim, D.; Kim, D.; Park, H.; Lee, S.-H.J.T.E. Engineering and Functionalization of Gelatin Biomaterials: From Cell Culture to Medical Applications. Tissue Eng. 2020. [Google Scholar] [CrossRef] [Green Version]
- Manoj, D.; Auddy, I.; Nimbkar, S.; Chittibabu, S.; Shanmugasundaram, S. Development of Screen-Printed Electrode Biosensor for Rapid Determination of Triglyceride Content in Coconut Milk. Int. J. Food Sci. 2020, 2020, 1696201. [Google Scholar] [CrossRef]
- Sung, H.W.; Huang, D.M.; Chang, W.H.; Huang, R.N.; Hsu, J.C. Evaluation of gelatin hydrogel crosslinked with various crosslinking agents as bioadhesives: In vitro study. J. Biomed. Mater. Res. 1999, 46, 520–530. [Google Scholar] [CrossRef]
- Kavoosi, G.; Bordbar, Z.; Dadfar, S.M.; Dadfar, S.M.M. Preparation and characterization of a novel gelatin–poly (vinyl alcohol) hydrogel film loaded with Zataria multiflora essential oil for antibacterial–antioxidant wound-dressing applications. J. Appl. Polym. Sci. 2017, 134, 45351. [Google Scholar] [CrossRef]
- Pan, T.; Song, W.; Cao, X.; Wang, Y. 3D bioplotting of gelatin/alginate scaffolds for tissue engineering: Influence of crosslinking degree and pore architecture on physicochemical properties. J. Mater. Sci. Technol. 2016, 32, 889–900. [Google Scholar] [CrossRef]
- Wang, C.; Yan, Q.; Liu, H.-B.; Zhou, X.-H.; Xiao, S.-J. Different EDC/NHS activation mechanisms between PAA and PMAA brushes and the following amidation reactions. Langmuir 2011, 27, 12058–12068. [Google Scholar] [CrossRef]
- Zhou, Q.; Chen, H.; Wang, Y. Region-selective electroless gold plating on polycarbonate sheets by UV-patterning in combination with silver activating. Electrochim. Acta 2010, 55, 2542–2549. [Google Scholar] [CrossRef]
- Renedo, O.D.; Alonso-Lomillo, M.A.; Martínez, M.J.A. Recent developments in the field of screen-printed electrodes and their related applications. Talanta 2007, 73, 202–219. [Google Scholar] [CrossRef] [PubMed]
- Taleat, Z.; Khoshroo, A.; Mazloum-Ardakani, M. Screen-printed electrodes for biosensing: A review (2008–2013). Microchim. Acta 2014, 181, 865–891. [Google Scholar] [CrossRef]
- Tudorache, M.; Bala, C. Biosensors based on screen-printing technology, and their applications in environmental and food analysis. Anal. Bioanal. Chem. 2007, 388, 565–578. [Google Scholar] [CrossRef]
- Arduini, F.; Micheli, L.; Moscone, D.; Palleschi, G.; Piermarini, S.; Ricci, F.; Volpe, G. Electrochemical biosensors based on nanomodified screen-printed electrodes: Recent applications in clinical analysis. TrAC Trends Anal. Chem. 2016, 79, 114–126. [Google Scholar] [CrossRef] [Green Version]
- Yamanaka, K.; Vestergaard, M.C.; Tamiya, E. Printable Electrochemical Biosensors: A Focus on Screen-Printed Electrodes and Their Application. Sensors (Basel Switz.) 2016, 16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, H.; Yang, J.; Wan, K.; Jiang, D.; Jin, C.-H. Miniaturized Paper-Supported 3D Cell-Based Electrochemical Sensor for Bacterial Lipopolysaccharide Detection. ACS Sens. 2020. [Google Scholar] [CrossRef]
- Cahn, G.; Barrios, A.; Graham, S.; Meth, J.; Antoniou, A.; Pierron, O. The role of strain localization on the electrical behavior of flexible and stretchable screen printed silver inks on polymer substrates. Materialia 2020, 10, 100642. [Google Scholar] [CrossRef]
- Hannah, S.; Al-Hatmi, M.; Gray, L.; Corrigan, D.K. Low-cost, thin-film, mass-manufacturable carbon electrodes for detection of the neurotransmitter dopamine. Bioelectrochemistry 2020, 133, 107480. [Google Scholar] [CrossRef]
- Alvarado, S.; Sandoval, G.; Palos, I.; Tellez, S.; Aguirre-Loredo, Y.; Velazquez, G. The effect of relative humidity on tensile strength and water vapor permeability in chitosan, fish gelatin and transglutaminase edible films. Food Sci. Technol. 2015, 35, 690–695. [Google Scholar] [CrossRef] [Green Version]
- Standard, A. D3359-97. In Standard Test Methods for Measuring Adhesion by Tape Test; ASTM International: West Conshohocken, PA, USA, 1997. [Google Scholar]
- Liu, X.; Rodeheaver, D.P.; White, J.C.; Wright, A.M.; Walker, L.M.; Zhang, F.; Shannon, S. A comparison of in vitro cytotoxicity assays in medical device regulatory studies. Regul. Toxicol. Pharmacol. 2018, 97, 24–32. [Google Scholar] [CrossRef]
- Talebian, A.; Kordestani, S.; Rashidi, A.; Dadashian, F.; Montazer, M. The Effect of Glutaraldehyde on the Properties of Gelatin Films. Kem. Ind. 2007, 56, 537–541. [Google Scholar]
- Iwanaga, K.; Yabuta, T.; Kakemi, M.; Morimoto, K.; Tabata, Y.; Ikada, Y. Usefulness of microspheres composed of gelatin with various cross-linking density. J. Microencapsul. 2003, 20, 767–776. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.C.; Chang, W.H.; Liang, H.F.; Lee, M.H.; Sung, H.W. Crosslinking structures of gelatin hydrogels crosslinked with genipin or a water-soluble carbodiimide. J. Appl. Polym. Sci. 2004, 91, 4017–4026. [Google Scholar] [CrossRef]
- Yang, G.; Xiao, Z.; Long, H.; Ma, K.; Zhang, J.; Ren, X.; Zhang, J. Assessment of the characteristics and biocompatibility of gelatin sponge scaffolds prepared by various crosslinking methods. Sci. Rep. 2018, 8, 1616. [Google Scholar] [CrossRef] [PubMed]






© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kang, P.-L.; Lin, Y.-H.; Settu, K.; Yen, C.-S.; Yeh, C.-Y.; Liu, J.-T.; Chen, C.-J.; Chang, S.-J. A Facile Fabrication of Biodegradable and Biocompatible Cross-Linked Gelatin as Screen Printing Substrates. Polymers 2020, 12, 1186. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051186
Kang P-L, Lin Y-H, Settu K, Yen C-S, Yeh C-Y, Liu J-T, Chen C-J, Chang S-J. A Facile Fabrication of Biodegradable and Biocompatible Cross-Linked Gelatin as Screen Printing Substrates. Polymers. 2020; 12(5):1186. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051186
Chicago/Turabian StyleKang, Pei-Leun, Yu-Hsin Lin, Kalpana Settu, Ching-Shu Yen, Chin-Yi Yeh, Jen-Tsai Liu, Ching-Jung Chen, and Shwu-Jen Chang. 2020. "A Facile Fabrication of Biodegradable and Biocompatible Cross-Linked Gelatin as Screen Printing Substrates" Polymers 12, no. 5: 1186. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051186