Development of Poly(l-Lactic Acid)-Based Bending Actuators
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Neat and Composite PLLA-Based Films
2.3. Characterization
2.3.1. Morphological, Structural and Thermal Analyses
2.3.2. Electrical and Electromechanical Characterization
3. Results and Discussion
3.1. Morphology
3.2. Physical-Chemical Characterization
3.3. Mechanical Properties
3.4. Electrical Properties
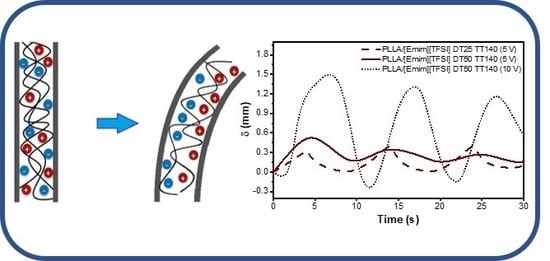
3.5. Electromechanical Measurements
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- Chorsi, M.T.; Curry, E.J.; Chorsi, H.T.; Das, R.; Baroody, J.; Purohit, P.K.; Ilies, H.; Nguyen, T.D. Piezoelectric Biomaterials for Sensors and Actuators. Adv. Mater. 2019, 31, 1802084. [Google Scholar] [CrossRef] [Green Version]
- Tyler, B.; Gullotti, D.; Mangraviti, A.; Utsuki, T.; Brem, H. Polylactic acid (PLA) controlled delivery carriers for biomedical applications. Adv. Drug Deliv. Rev. 2016, 107, 163–175. [Google Scholar] [CrossRef] [PubMed]
- Hyon, S.H. Biodegradable poly (lactic acid) microspheres for drug delivery systems. Yonsei Med. J. 2000, 41, 720–734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, F.; Murugan, R.; Ramakrishna, S.; Wang, X.; Ma, Y.X.; Wang, S. Fabrication of nano-structured porous PLLA scaffold intended for nerve tissue engineering. Biomaterials 2004, 25, 1891–1900. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, C.; Sencadas, V.; Correia, D.M.; Lanceros-Méndez, S. Piezoelectric polymers as biomaterials for tissue engineering applications. Colloids Surf. B Biointerfaces 2015, 136, 46–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, D.M.; Sencadas, V.; Ribeiro, C.; Martins, P.M.; Martins, P.; Gama, F.M.; Botelho, G.; Lanceros-Méndez, S. Processing and size range separation of pristine and magnetic poly(l-lactic acid) based microspheres for biomedical applications. J. Colloid Interface Sci. 2016, 476, 79–86. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curry, E.J.; Ke, K.; Chorsi, M.T.; Wrobel, K.S.; Miller, A.N.; Patel, A.; Kim, I.; Feng, J.; Yue, L.; Wu, Q.; et al. Biodegradable Piezoelectric Force Sensor. Proc. Natl. Acad. Sci. USA 2018, 115, 909. [Google Scholar] [CrossRef] [Green Version]
- Tajitsu, Y. Fundamental study on improvement of piezoelectricity of poly(ι-lactic acid) and its application to film actuators. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 2013, 60, 1625–1629. [Google Scholar] [CrossRef]
- Noshadi, I.; Walker, B.W.; Portillo-Lara, R.; Shirzaei Sani, E.; Gomes, N.; Aziziyan, M.R.; Annabi, N. Engineering Biodegradable and Biocompatible Bio-ionic Liquid Conjugated Hydrogels with Tunable Conductivity and Mechanical Properties. Sci. Rep. 2017, 7, 4345. [Google Scholar] [CrossRef] [Green Version]
- Ye, Y.-S.; Rick, J.; Hwang, B.-J. Ionic liquid polymer electrolytes. J. Mater. Chem. A 2013, 1, 2719–2743. [Google Scholar] [CrossRef]
- Dong, K.; Liu, X.; Dong, H.; Zhang, X.; Zhang, S. Multiscale Studies on Ionic Liquids. Chem. Rev. 2017, 117, 6636–6695. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.G.; Zhang, Q.H.; Zhang, Y.; Chen, Z.J.; Watanabe, M.; Deng, Y.Q. Beyond solvents and electrolytes: Ionic liquids-based advanced functional materials. Prog. Mater. Sci. 2016, 77, 80–124. [Google Scholar] [CrossRef] [Green Version]
- Lei, Z.; Chen, B.; Koo, Y.-M.; MacFarlane, D.R. Introduction: Ionic Liquids. Chem. Rev. 2017, 117, 6633–6635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, P.; Cui, Z.-P.; Ruan, G.; Ding, Y.-S. Enhanced Crystallization Kinetics of PLLA by Ethoxycarbonyl Ionic Liquid Modified Graphene. Chin. J. Polym. Sci. 2019, 37, 243–252. [Google Scholar] [CrossRef]
- Correia, D.M.; Fernandes, L.C.; Martins, P.M.; García-Astrain, C.; Costa, C.M.; Reguera, J.; Lanceros-Méndez, S. Ionic Liquid–Polymer Composites: A New Platform for Multifunctional Applications. Adv. Funct. Mater. 2020, 1909736. [Google Scholar] [CrossRef]
- Yoo, C.G.; Pu, Y.Q.; Ragauskas, A.J. Ionic liquids: Promising green solvents for lignocellulosic biomass utilization. Curr. Opin. Green Sustain. Chem. 2017, 5, 5–11. [Google Scholar] [CrossRef]
- Marr, P.C.; Marr, A.C. Ionic liquid gel materials: applications in green and sustainable chemistry. Green Chem. 2016, 18, 105–128. [Google Scholar] [CrossRef] [Green Version]
- Mejri, R.; Dias, J.C.; Hentati, S.B.; Botelho, G.; Esperanca, J.; Costa, C.M.; Lanceros-Mendez, S. Imidazolium-based ionic liquid type dependence of the bending response of polymer actuators. Eur. Polym. J. 2016, 85, 445–451. [Google Scholar] [CrossRef]
- Dias, J.C.; Correia, D.M.; Costa, C.M.; Ribeiro, C.; Maceiras, A.; Vilas, J.L.; Botelho, G.; de Zea Bermudez, V.; Lanceros-Mendez, S. Improved response of ionic liquid-based bending actuators by tailored interaction with the polar fluorinated polymer matrix. Electrochim. Acta 2019, 296, 598–607. [Google Scholar] [CrossRef]
- Terasawa, N. High-performance transparent actuator made from Poly(dimethylsiloxane)/Ionic liquid gel. Sens. Actuators B-Chem. 2018, 257, 815–819. [Google Scholar] [CrossRef]
- Wei, T.; Pang, S.; Xu, N.; Pan, L.; Zhang, Z.; Xu, R.; Ma, N.; Lin, Q. Crystallization behavior and isothermal crystallization kinetics of PLLA blended with ionic liquid, 1-butyl-3-methylimidazolium dibutylphosphate. J. Appl. Polym. Sci. 2015, 132, 41308. [Google Scholar] [CrossRef]
- Gui, H.; Li, Y.; Chen, S.; Xu, P.; Zheng, B.; Ding, Y. Effects of biodegradable imidazolium-based ionic liquid with ester group on the structure and properties of PLLA. Macromol. Res. 2014, 22, 583–591. [Google Scholar] [CrossRef]
- Gardella, L.; Furfaro, D.; Galimberti, M.; Monticelli, O. On the development of a facile approach based on the use of ionic liquids: preparation of PLLA (sc-PLA)/high surface area nano-graphite systems. Green Chem. 2015, 17, 4082–4088. [Google Scholar] [CrossRef]
- Correia, D.M.; Barbosa, J.C.; Costa, C.M.; Reis, P.M.; Esperança, J.M.S.S.; de Zea Bermudez, V.; Lanceros-Méndez, S. Ionic Liquid Cation Size-Dependent Electromechanical Response of Ionic Liquid/Poly(vinylidene fluoride)-Based Soft Actuators. J. Phys. Chem. C 2019, 123, 12744–12752. [Google Scholar] [CrossRef]
- Ribeiro, C.; Sencadas, V.; Costa, C.M.; Gómez Ribelles, J.L.; Lanceros-Méndez, S. Tailoring the morphology and crystallinity of poly(L-lactide acid) electrospun membranes. Sci. Technol. Adv. Mater. 2011, 12, 015001. [Google Scholar] [CrossRef]
- Mazumder, M.; Ahmed, R.; Wajahat Ali, A.; Lee, S.-J. SEM and ESEM techniques used for analysis of asphalt binder and mixture: A state of the art review. Constr. Build. Mater. 2018, 186, 313–329. [Google Scholar] [CrossRef]
- Ribeiro, C.; Costa, C.M.; Correia, D.M.; Nunes-Pereira, J.; Oliveira, J.; Martins, P.; Gonçalves, R.; Cardoso, V.F.; Lanceros-Méndez, S. Electroactive poly(vinylidene fluoride)-based structures for advanced applications. Nat. Protoc. 2018, 13, 681–704. [Google Scholar] [CrossRef]
- Kawai, T.; Rahman, N.; Matsuba, G.; Nishida, K.; Kanaya, T.; Nakano, M.; Okamoto, H.; Kawada, J.; Usuki, A.; Honma, N.; et al. Crystallization and Melting Behavior of Poly (l-lactic Acid). Macromolecules 2007, 40, 9463–9469. [Google Scholar] [CrossRef]
- Krikorian, V.; Pochan, D.J. Crystallization Behavior of Poly(l-lactic acid) Nanocomposites: Nucleation and Growth Probed by Infrared Spectroscopy. Macromolecules 2005, 38, 6520–6527. [Google Scholar] [CrossRef]
- Cao, X.; Mohamed, A.; Gordon, S.H.; Willett, J.L.; Sessa, D.J. DSC study of biodegradable poly(lactic acid) and poly(hydroxy ester ether) blends. Thermochim. Acta 2003, 406, 115–127. [Google Scholar] [CrossRef]
- Kiefer, J.; Fries, J.; Leipertz, A. Experimental Vibrational Study of Imidazolium-Based Ionic Liquids: Raman and Infrared Spectra of 1-Ethyl-3-methylimidazolium Bis(Trifluoromethylsulfonyl)imide and 1-Ethyl-3-methylimidazolium Ethylsulfate. Appl. Spectrosc. 2007, 61, 1306–1311. [Google Scholar] [CrossRef]
- Jiao, Q.; Chen, Q.; Wang, L.; Chen, H.L.; Li, Y.J. Investigation on the Crystallization Behaviors of Polyoxymethylene with a Small Amount of Ionic Liquid. Nanomaterials 2019, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, D.M.; Costa, C.M.; Lizundia, E.; Sabater i Serra, R.; Gómez-Tejedor, J.A.; Biosca, L.T.; Meseguer-Dueñas, J.M.; Gomez Ribelles, J.L.; Lanceros-Méndez, S. Influence of Cation and Anion Type on the Formation of the Electroactive β-Phase and Thermal and Dynamic Mechanical Properties of Poly(vinylidene fluoride)/Ionic Liquids Blends. J. Phys. Chem. C 2019, 123, 27917–27926. [Google Scholar] [CrossRef]
- Mirkhalaf, S.M.; Fagerström, M. The mechanical behavior of polylactic acid (PLA) films: fabrication, experiments and modelling. Mech. Time-Depend. Mater. 2019. [Google Scholar] [CrossRef] [Green Version]
- Aziz, S.B.; Woo, T.J.; Kadir, M.F.Z.; Ahmed, H.M. A conceptual review on polymer electrolytes and ion transport models. J. Sci. Adv. Mater. Devices 2018, 3, 1–17. [Google Scholar] [CrossRef]
- Reizabal, A.; Correia, D.M.; Costa, C.M.; Perez-Alvarez, L.; Vilas-Vilela, J.L.; Lanceros-Méndez, S. Silk Fibroin Bending Actuators as an Approach Toward Natural Polymer Based Active Materials. ACS Appl. Mater. Interfaces 2019, 11, 30197–30206. [Google Scholar] [CrossRef]










| Wavenumbers (cm−1) | Attribution | |
|---|---|---|
| PLLA [29] | [Emim][TFSI] [32] | |
| 2997 | CH3 asymmetric stretching | - |
| 1752 | C=O stretching | - |
| 1570 | - | ring vibration N–CH3 and N–CH2–CH3 |
| 1454 | CH3 asymmetric stretching | - |
| 1383 | CH3 symmetric stretching | - |
| 1358 | CH stretching+CH3 symmetric stretching | - |
| 1348 | SO2 symmetric stretching | |
| 1300 | CH stretching | - |
| 1184 | C–O–C +CH3 asymmetric stretching | CF3 antisymmetric stretching |
| 1132 | CH3 asymmetric stretching | SO2 symmetric stretching |
| 1085 | C–O–C symmetric stretching | - |
| 1045 | C–CH3 stretching | |
| 1051 | C–C and NCH3 stretching | |
| 921 | C–C stretching + CH3 rotation | - |
| 871 | C–COO stretching | |
| 738 | - | CF3 stretching |
| 755 | C=O stretching | - |
| 740 | CF3 symmetric stretching | |
| 651 | S–N–S bending | |
| 619 | - | SO2 antisymmetric bending |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
M. Correia, D.; C. Fernandes, L.; Cruz, B.D.D.; Botelho, G.; de Zea Bermudez, V.; Lanceros-Méndez, S. Development of Poly(l-Lactic Acid)-Based Bending Actuators. Polymers 2020, 12, 1187. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051187
M. Correia D, C. Fernandes L, Cruz BDD, Botelho G, de Zea Bermudez V, Lanceros-Méndez S. Development of Poly(l-Lactic Acid)-Based Bending Actuators. Polymers. 2020; 12(5):1187. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051187
Chicago/Turabian StyleM. Correia, Daniela, Liliana C. Fernandes, Bárbara D.D. Cruz, Gabriela Botelho, Verónica de Zea Bermudez, and Senentxu Lanceros-Méndez. 2020. "Development of Poly(l-Lactic Acid)-Based Bending Actuators" Polymers 12, no. 5: 1187. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12051187