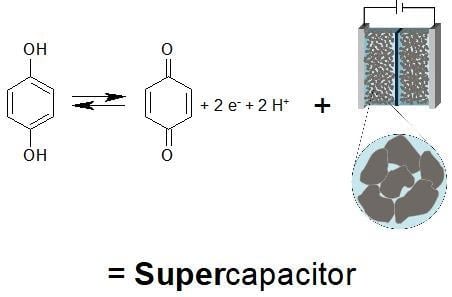
Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes—An Introduction †
Abstract
:1. Introduction
2. Quinones in Supercapacitors
3. Ferrocenes in Supercapacitors
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Dubal, D.P.; Wu, Y.; Holze, R. Supercapacitors as fast storage systems for electric energy. Bunsen Mag. 2015, 17, 216–227. [Google Scholar]
- Shukla, A.K.; Sampath, S.; Vijayamohanan, K. Electrochemical supercapacitors: Energy storage beyond batteries. Curr. Sci. 2000, 79, 1656–1661. [Google Scholar]
- Dubal, D.P.; Wu, Y.P.; Holze, R. Supercapacitors: From the Leyden jar to electric busses. ChemTexts 2016, 2, 13. [Google Scholar] [CrossRef] [Green Version]
- Conway, B.E. Electrochemical Supercapacitors: Scientific Fundamentals and Technological Applications; Springer: New York, NY, USA, 1999. [Google Scholar]
- Yu, A.; Chabot, V.; Zhang, J. Electrochemical Supercapacitors for Energy Storage and Delivery—Fundamentals and Applications; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar]
- Inamuddin; Ahmer, M.F.; Asiri, A.M.; Zaidi, S. Electrochemical Capacitors in: Materials Research Foundations; Materials Research Forum LLC: Millersville, PA, USA, 2018; Volume 26. [Google Scholar]
- Stevic, Z. Supercapacitor Design and Applications; IntechOpen: London, UK, 2016; ExLi4EvA2016. [Google Scholar]
- Beguin, F.; Frackowiak, E. Supercapacitors; Wiley-VCH: Weinheim, Germany, 2013. [Google Scholar]
- Miller, J.M. Ultracapacitor Applications; The Institution of Engineering and Technology: London, UK, 2011. [Google Scholar]
- Conway, B.E.; Gileadi, E. Kinetic theory of pseudo-capacitance and electrode reactions at appreciable surface coverage. Trans. Faraday Soc. 1962, 58, 2493–2509. [Google Scholar] [CrossRef]
- Wu, Y.; Holze, R. Battery and/or Supercapacitor?—On the merger of two electrochemical storage families. ChemTexts, in preparation.
- Simon, P.; Gogotsi, Y.; Dunn, B. Where do batteries end and supercapacitors begin? Science 2014, 343, 1210–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zuo, W.; Li, R.; Zhou, C.; Li, Y.; Xia, J.; Liu, J. Battery-supercapacitor hybrid devices: Recent progress and future prospects. Adv. Sci. 2017, 4, 1600539. [Google Scholar] [CrossRef] [PubMed]
- Chen, G.Z. Supercapacitor and supercapattery as emerging electrochemical energy stores. Int. Mater. Rev. 2017, 62, 173–202. [Google Scholar] [CrossRef] [Green Version]
- Pan, H.-A.; Ghodbane, O.; Weng, Y.-T.; Sheu, H.-S.; Lee, J.-F.; Favier, F.; Wu, N.-L. Investigating mechanisms underlying elevated-temperature-induced capacity fading of aqueous MnO2 polymorph supercapacitors: Cryptomelane and birnessite. J. Electrochem. Soc. 2015, 162, A5106–A5114. [Google Scholar] [CrossRef]
- Otero, T.F.; Cantero, I. Conducting polymers as positive electrodes in rechargeable lithium-ion batteries. J. Power Sources 1999, 81–82, 838–841. [Google Scholar] [CrossRef]
- Kita, T.; Daifuku, H.; Fujio, R.; Fuse, T.; Kawagoe, T.; Masuda, Y.; Matsunaga, T.; Ogawa, M. Properties of polyaniline secondary battery. J. Electrochem. Soc. 1986, 133, C291. [Google Scholar]
- Matsunaga, T.; Daifuku, H.; Kawagoe, T. Development of polyaniline-lithium secondary battery. Nippon Kagaku Kaishi 1990, 1990, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Daniel, C.; Besenhard, J.O. Handbook of Battery Materials; Wiley-VCH: Weinheim, Germany, 2011. [Google Scholar]
- Mammone, R.J. Use of electronically conducting polymers as catalytic electrodes in aqueous and inorganic electrolytes. In Conducting Polymers—Special Applications; Alcacer, L., Ed.; D. Reidel Publishing Company: Dordrecht, The Netherlands, 1987; pp. 161–172. [Google Scholar]
- Koga, K.; Yamasaki, S.; Narimatsu, K.; Takayanagi, M. Electrically conductive composite of polyaniline aramid and its application as a cathode material for secondary battery. Polym. J. 1989, 21, 733–738. [Google Scholar] [CrossRef] [Green Version]
- Rudge, A.; Davey, J.; Raistrick, I.; Gottesfeld, S.; Ferraris, J.P. Conducting polymers as active materials in electrochemical capacitors. J. Power Sources 1994, 47, 89–107. [Google Scholar] [CrossRef]
- Conway, B.E. Transition from “Supercapacitor” to “Battery” behavior in electrochemical energy storage. J. Electrochem. Soc. 1991, 138, 1539–1548. [Google Scholar] [CrossRef]
- Holze, R.; Wu, Y.P. Intrinsically conducting polymers in electrochemical energy technology: Trends and progress. Electrochim. Acta 2014, 122, 93–107. [Google Scholar] [CrossRef]
- Novak, P.; Müller, K.; Santhanam, K.S.V.; Haas, O. Electrochemically active polymers for rechargeable batteries. Chem. Rev. 1997, 97, 207–281. [Google Scholar] [CrossRef]
- Fong, K.D.; Wang, T.; Smoukov, S.K. Multidimensional performance optimization of conducting polymer-based supercapacitor electrodes. Sustain. Energy Fuels 2017, 1, 1857–1874. [Google Scholar] [CrossRef] [Green Version]
- Holze, R. Metal oxide/conducting polymer hybrids for application in supercapacitors. In Metal Oxides in Supercapacitors; Dubal, D.P., Gomez-Romero, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 219–245. [Google Scholar]
- Fu, L.; Qu, Q.; Holze, R.; Kondratiev, V.V.; Wu, Y. Composites of metal oxides and intrinsically conducting polymers as supercapacitor electrodes: The best of both worlds? J. Mater. Chem. A 2019, 7, 14937–14970. [Google Scholar] [CrossRef]
- Armand, M.; Tarascon, J.-M. Building better batteries. Nature 2008, 451, 652–657. [Google Scholar] [CrossRef]
- Chen, H.Y.; Armand, M.; Courty, M.; Jiang, M.; Grey, C.P.; Dolhem, F.; Tarascon, J.M.; Poizot, P. Lithium Salt of Tetrahydroxybenzoquinone: Toward the development of a Sustainable Li-Ion Battery. J. Am. Chem. Soc. 2009, 131, 8984–8988. [Google Scholar] [CrossRef]
- Armand, M.; Grugeon, S.; Vezin, H.; Laruelle, S.; Ribiere, P.; Poizot, P.; Tarascon, J.-M. Conjugated dicarboxylate anodes for Li-ion batteries. Nat. Mater. 2009, 8, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Walker, W.; Grugeon, S.; Vezin, H.; Laruelle, S.; Armand, M.; Tarascon, J.M.; Wudl, F. The effect of length and cis/trans relationship of conjugated pathway on secondary battery performance in organolithium electrodes. Electrochem. Commun. 2010, 12, 1348–1351. [Google Scholar] [CrossRef]
- Chen, H.; Armand, M.; Demailly, G.; Dolhem, F.; Poizot, P.; Tarascon, J.M. From biomass to a renewable LixC6O6 organic electrode forsustainable Li-ion batteries. Chem. Sus. Chem. 2008, 1, 348–355. [Google Scholar] [CrossRef] [PubMed]
- Algharaibeh, Z.; Liu, X.R.; Pickup, P.G. An asymmetric anthraquinone-modified carbon/ruthenium oxide supercapacitor. J. Power Sources 2009, 187, 640–643. [Google Scholar] [CrossRef]
- Admassie, S.; Ajjan, F.N.; Elfwing, A.; Inganäs, O. Biopolymer hybrid electrodes for scalable electricity storage. Mater. Horiz. 2016, 3, 174–185. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Holze, R. Electrocatalysis at electrodes for vanadium redox flow batteries. Batteries 2018, 4, 47. [Google Scholar] [CrossRef] [Green Version]
- Algharaibeh, Z.; Pickup, P.G. An asymmetric supercapacitor with anthraquinone and dihydroxybenzene modified carbon fabric electrodes. Electrochem. Commun. 2011, 13, 147–149. [Google Scholar] [CrossRef]
- Kalinathan, K.; DesRoches, D.P.; Liu, X.; Pickup, P.G. Anthraquinone modified carbon fabric supercapacitors with improved energy and power densities. J. Power Sources 2008, 181, 182–185. [Google Scholar] [CrossRef]
- Smith, R.D.L.; Pickup, P.G. Novel electroactive surface functionality from the coupling of an aryl diamine to carbon black. Electrochem. Commun. 2009, 11, 10–13. [Google Scholar] [CrossRef]
- Medany, S.S.; Ismail, K.M.; Badawy, W.A. Kinetics of the electropolymerization of aminoanthraquinone from aqueous solutions and analytical applications of the polymer film. J. Adv. Res. 2012, 3, 261–268. [Google Scholar] [CrossRef] [Green Version]
- Badawy, W.A.; Ismail, K.M.; Medany, S.S. Kinetics of Electropolymerization of 1-Amino-9, 10-anthraquinone. Int. J. Chem. Kinet. 2011, 43, 141–146. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Medany, S.S. Optimization of the electropolymerization of 1-amino-9,10-anthraquinone conducting films from aqueous media. Electrochim. Acta 2006, 51, 6353–6360. [Google Scholar] [CrossRef]
- Ismail, K.M.; Khalifa, Z.M.; Azzem, M.A.; Badawy, W.A. Electrochemical preparation and characterization of poly(1-amino-9,10-anthraquinone) films. Electrochim. Acta 2002, 47, 1867–1873. [Google Scholar] [CrossRef]
- Badawy, W.A.; Ismail, K.M.; Medany, S.S. Electropolymerization of Aminoanthraquinone from H2SO4/Acetonitrile Mixed Solvent—Polymer Film Characterization and its Use as Analytical Sensor. Z. Phys. Chem. 2013, 227, 1741–1757. [Google Scholar] [CrossRef]
- Algharaibeh, Z.; Pickup, P.G. Charge trapping in poly(1-amino-anthraquinone) films. Electrochim. Acta 2013, 93, 87–92. [Google Scholar] [CrossRef]
- Holze, R. Copolymers—A refined way to tailor intrinsically conducting polymers. Electrochim. Acta 2011, 56, 10479–10492. [Google Scholar] [CrossRef]
- Yamamoto, K.; Yamada, M.; Nishiumi, T. Doping reaction of redox-active dopants into polyaniline. Polym. Adv. Technol. 2000, 11, 710–715. [Google Scholar] [CrossRef]
- Navarro-Suárez, A.M.; Casado, N.; Carretero-Gonzalez, J.; Mecerreyes, D.; Rojo, T. Full-cell quinone/hydroquinone supercapacitors based on partially reduced graphite oxide and lignin/PEDOT electrodes. J. Mater. Chem. A 2017, 5, 7137–7143. [Google Scholar] [CrossRef]
- Ren, L.; Zhang, G.; Lei, J.; Wang, Y.; Hu, D. Novel layered polyaniline-poly(hydroquinone)/graphene film as supercapacitor electrode with enhanced rate performance and cycling stability. J. Colloid Interface Sci. 2018, 512, 300–307. [Google Scholar] [CrossRef]
- Stejskal, J.; Bober, P.; Trchova, M.; Horsky, J.; Pilar, J.; Walterova, Z. The oxidation of aniline with p-benzoquinone and its impact on the preparation of the conducting polymer, polyaniline. Synth. Met. 2014, 192, 66–73. [Google Scholar] [CrossRef]
- Vonlanthen, D.; Lazarev, P.; See, K.A.; Wudl, F.; Heeger, A.J. A Stable polyaniline-benzoquinone-hydroquinone supercapacitor. Adv. Mater. 2014, 26, 5095–5100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malinauskas, A.; Holze, R. Electrocatalysis of the quinone/hydroquinone system by electrodes coated with substituted polyaniline. Ber. Bunsenges. Phys. Chem. 1996, 100, 1740–1745. [Google Scholar] [CrossRef]
- Büttner, E.; Holze, R. Elektrokatalyse von Redoxreaktionen an leitfähigen polymeren. In GDCh-Monographie; Russow, J., Schäfer, J.H., Eds.; GDCh: Frankfurt, Germany, 2002; Volume 23, pp. 209–216. [Google Scholar]
- Büttner, E.; Holze, R. Hydroquinone oxidation electrocatalysis at polyaniline films. J. Electroanal. Chem. 2001, 508, 150–155. [Google Scholar] [CrossRef]
- Gao, L.; Gan, S.; Li, H.; Han, D.; Li, F.; Bao, Y.; Niu, L. Self-assembling graphene-anthraquinone-2-sulphonate supramolecular nanostructures with enhanced energy density for supercapacitors. Nanotechnology 2017, 28, 275602. [Google Scholar] [CrossRef] [PubMed]
- Fernandez, J.; Bonastre, J.; Molina, J.; Cases, F. Electrochemical study on an activated carbon cloth modified by cyclic voltammetry with polypyrrole/anthraquinone sulfonate and reduced graphene oxide as electrode for energy storage. Eur. Polym. J. 2018, 103, 179–186. [Google Scholar] [CrossRef]
- Wan, Q.-Y.; Fan, S.-S.; Yue, X.-J.; Lang, X.-M.; Xu, W.-D.; Li, J.; Feng, C.-H. Improved Capacitive Performance of Polypyrrole Doped with 9,10-Anthraquinone-2-sulfonic Acid Sodium Salt. Acta Phys. Chim. Sin. 2010, 26, 2951–2956. [Google Scholar]
- Yoneyama, H.; Li, Y.; Kuwabata, S. Charge-discharge characteristics of polypyrrole films containing incorporated anthraquinone-1-sulfonate. J. Electrochem. Soc. 1992, 139, 28–32. [Google Scholar] [CrossRef]
- Lang, X.; Wan, Q.; Feng, C.; Yue, X.; Xu, W.; Li, J.; Fan, S. The role of anthraquinone sulfonate dopants in promoting performance of polypyrrole composites as pseudo-capacitive electrode materials. Synth. Met. 2010, 160, 1800–1804. [Google Scholar] [CrossRef]
- Kumar, A.S.; Swetha, P. Simple adsorption of anthraquinone on carbon nanotube modified electrode and its efficient electrochemical behaviors. Colloids Surf. A 2011, 384, 597–604. [Google Scholar] [CrossRef]
- Brousse, T.; Cougnon, C.; Belanger, D. Grafting of quinones on carbons as active electrode materials in electrochemical capacitors. J. Brazil. Chem. Soc. 2018, 29, 989–997. [Google Scholar] [CrossRef]
- Wang, G.; Feng, C. Electrochemical Polymerization of Hydroquinone on graphite felt as a pseudocapacitive material for application in a microbial fuel cell. Polymers 2017, 9, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Wang, B.; Liu, C.; Han, N.; Xu, X.; Zhao, F.; Fan, J.; Li, Y. Polyanthraquinone-based nanostructured electrode material capable of high-performance pseudocapacitive energy storage in aprotic electrolyte. Nano Energy 2015, 15, 654–661. [Google Scholar] [CrossRef]
- Pognon, G.; Cougnon, C.; Mayilukila, D.; Bélanger, D. Catechol-modified activated carbon prepared by the diazonium chemistry for application as active electrode material in electrochemical capacitor. ACS Appl. Mater. Inter. 2012, 4, 3788–3796. [Google Scholar] [CrossRef] [PubMed]
- Cougnon, C.; Lebègue, E.; Pognon, G. Impedance spectroscopy study of a catechol-modified activated carbon electrode as active material in electrochemical capacitor. J. Power Sources 2015, 274, 551–559. [Google Scholar] [CrossRef]
- Malka, D.; Hanna, O.; Hauser, T.; Hayne, S.; Luski, S.; Elias, Y.; Attias, R.; Brousse, T.; Aurbach, D. Improving the capacity of electrochemical capacitor electrode by grafting 2-aminoanthraquinone over kynol carbon cloth using diazonium chemistry. J. Electrochem. Soc. 2018, 165, A3342–A3349. [Google Scholar] [CrossRef]
- Weissmann, M.; Crosnier, O.; Brousse, T.; Bélanger, D. Electrochemical study of anthraquinone groups, grafted by the diazonium chemistry, in different aqueous media-relevance for the development of aqueous hybrid electrochemical capacitor. Electrochim. Acta 2012, 82, 250–256. [Google Scholar] [CrossRef]
- Pognon, G.; Brousse, T.; Demarconnay, L.; Bélanger, D. Performance and stability of electrochemical capacitor based on anthraquinone modified activated carbon. J. Power Sources 2011, 196, 4117–4122. [Google Scholar] [CrossRef]
- Le Comte, A.; Pognon, G.; Brousse, T.; Bélanger, D. Determination of the quinone-loading of a modified carbon powder-based electrode for electrochemical capacitor. Electrochemistry 2013, 81, 863–866. [Google Scholar] [CrossRef] [Green Version]
- Le Comte, A.; Brousse, T.; Bélanger, D. Chloroanthraquinone as a grafted probe molecule to investigate grafting yield on carbon powder. Electrochim. Acta 2016, 197, 139–145. [Google Scholar] [CrossRef]
- Le Comte, A.; Brousse, T.; Bélanger, D. Simpler and greener grafting method for improving the stability of anthraquinone-modified carbon electrode in alkaline media. Electrochim. Acta 2014, 137, 447–453. [Google Scholar] [CrossRef]
- Le Comte, A.; Brousse, T.; Bélanger, D. New generation of hybrid carbon/Ni(OH)2 electrochemical capacitor using functionalized carbon electrode. J. Power Sources 2016, 326, 702–710. [Google Scholar] [CrossRef]
- Le Comte, A.; Chhin, D.; Gagnon, A.; Retoux, R.; Brousse, T.; Bélanger, D. Spontaneous grafting of 9,10-phenanthrenequinone on porous carbon as an active electrode material in an electrochemical capacitor in an alkaline electrolyte. J. Mater. Chem. A 2015, 3, 6146–6156. [Google Scholar] [CrossRef]
- Katsuyama, Y.; Nakayasu, Y.; Oizumi, K.; Fujihara, Y.; Kobayashi, H.; Honma, I. Quinone-based redox supercapacitor using highly conductive hard carbon derived from oak wood. Adv. Sust. Syst. 2019, 3, 1900083. [Google Scholar] [CrossRef]
- Tisawat, N.; Samart, C.; Jaiyong, P.; Bryce, R.A.; Nuengnoraj, K.; Chanlek, N.; Kongparakul, S. Enhancement performance of carbon electrode for supercapacitors by quinone derivatives loading via solvent-free method. Appl. Surf. Sci. 2019, 491, 784–791. [Google Scholar] [CrossRef]
- Ma, G.; Hua, F.; Sun, K.; Feng, E.; Zhang, Z.; Peng, H.; Lei, Z. Anthraquinones-modified porous carbon as composite electrode for symmetric supercapacitor. Ionics 2018, 24, 549–561. [Google Scholar] [CrossRef]
- Gao, X.; Du, D.; Li, S.; Yan, X.; Xing, W.; Bai, P.; Xue, Q.; Yan, Z. Outstanding capacitive performance of ordered mesoporous carbon modified by anthraquinone. Electrochim. Acta 2018, 259, 110–121. [Google Scholar] [CrossRef]
- Kumaran, K.T.; Kiruthika, G.V.M.; Arulraj, I.; Ragupathy, P. Quinone-wrapped nanostructured MnO2: A synergetic approach to enhanced supercapacitive behavior and magnetic properties. J. Electrochem. Soc. 2016, 163, A1743–A1752. [Google Scholar] [CrossRef]
- Leitner, K.W.; Gollas, B.; Winter, M.; Besenhard, J.O. Combination of redox capacity and double layer capacitance in composite electrodes through immobilization of an organic redox couple on carbon black. Electrochim. Acta 2004, 50, 199–204. [Google Scholar] [CrossRef]
- Gastol, D.; Walkowiak, J.; Fic, K.; Frackowiak, E. Enhancement of the carbon electrode capacitance by brominated hydroquinones. J. Power Sources 2016, 326, 587–594. [Google Scholar] [CrossRef] [Green Version]
- Frackowiak, E.; Meller, M.; Menzel, J.; Gastol, D.; Fic, K. Redox-active electrolyte for supercapacitor application. Faraday Discuss. 2014, 172, 179–198. [Google Scholar] [CrossRef]
- Roldán, S.; Blanco, C.; Granda, M.; Menéndez, M.; Santamaria, R. Towards a further generation of high-energy carbon-based capacitors by using redox-active electrolytes. Angew. Chem. Int. Ed. 2011, 50, 1699–1701. [Google Scholar] [CrossRef] [PubMed]
- Meller, M.; Fic, K. Effect of benzoquinone additives on the performance of symmetric carbon/carbon capacitors—Electrochemical impedance study. J. Energy Stor. 2018, 18, 340–348. [Google Scholar] [CrossRef]
- Zhu, Y.; Liu, E.; Luo, Z.; Hu, T.; Liu, T.; Li, Z.; Zhao, Q. A hydroquinone redox electrolyte for polyaniline/SnO2 supercapacitors. Electrochim. Acta 2014, 118, 106–111. [Google Scholar]
- Shul, G.; Bélanger, D. Self-discharge of electrochemical capacitors based on soluble or grafted quinone. Phys. Chem. Chem. Phys. 2016, 18, 19137–19145. [Google Scholar] [CrossRef] [PubMed]
- Han, Y.; Wang, T.; Li, T.; Gao, X.; Li, W.; Zhang, Z.; Wang, Y.; Zhang, X. Preparation and electrochemical performances of graphene/polypyrrole nanocomposite with anthraquinone-graphene oxide as active oxidant. Carbon 2017, 119, 111–118. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, X.; Zhang, S.; Chen, G.Z. Electrochemical considerations in supercapacitors with nanocomposites. ECS Transactions 2011, 33, 107–116. [Google Scholar] [CrossRef]
- Peng, C.; Zhou, X.; Chen, G.Z.; Moggia, F.; Fages, F.; Brisset, H.; Roncali, J. Internally referenced analysis of charge-transfer reactions in a new ferrocenyl bithiophenic conducting polymer through cyclic voltammetry. Chem. Commun. 2008, 2008, 6606–6608. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y. Preparation of a P(FcA-co-ANI)/graphene composite for application in supercapacitors. High. Perform. Polym. 2017, 29, 524–532. [Google Scholar] [CrossRef]
- Li, Y.; Zheng, Y. Synthesis and characterization of a ferrocene-modified, polyaniline-like conducting polymer. J. Appl. Polym. Sci. 2016, 133, 43217. [Google Scholar] [CrossRef]
- Zhang, X.; Ho, L.; Richard, F.; Samori, P. Modular preparation of graphene-based functional architectures through two-step organic reactions: Towards high-performance energy storage. Chem. Eur. J. 2018, 24, 18518–18528. [Google Scholar] [CrossRef]
- De Adhikari, A.; Oraon, R.; Tiwari, S.K.; Jena, N.K.; Lee, J.H.; Kim, N.H.; Nayak, G.C. Polyaniline-stabilized intertwined network-like ferrocene/graphene nanoarchitecture for supercapacitor application. Chem. Asian J. 2017, 12, 900–909. [Google Scholar] [CrossRef] [PubMed]
- Rahimpour, K.; Teimuri-Mofrad, R. Novel hybrid supercapacitor based on ferrocenyl modified graphene quantum dot and polypyrrole nanocomposite. Electrochim. Acta 2020, 345, 136207. [Google Scholar] [CrossRef]
- Tian, W.; Mao, X.; Brown, P.; Rutledge, G.C.; Hatton, T.A. Electrochemically nanostructured polyvinylferrocene/polypyrrole hybrids with synergy for energy storage. Adv. Funct. Mater. 2015, 25, 4803–4813. [Google Scholar] [CrossRef]
- Chow, K.-F.; Sardar, R.; Sassin, M.B.; Wallace, J.M.; Feldberg, S.W.; Rolison, D.R.; Long, J.W.; Murray, R.W. 3D-addressable redox: Modifying porous carbon electrodes with ferrocenated 2 nm gold nanoparticles. J. Phys. Chem. C 2012, 116, 9283–9289. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Abbasi, H.; Hadi, R. Graphene oxide-grafted ferrocene moiety via ring opening polymerization (ROP) as a supercapacitor electrode material. Polymer 2019, 167, 138–145. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Abbasi, H. Synthesis and characterization of ferrocene-functionalized reduced graphene oxide nanocomposite as a supercapacitor electrode material. J. Organomet. Chem. 2019, 880, 355–362. [Google Scholar] [CrossRef]
- Hadi, R.; Abbasi, H.; Payami, E.; Ahadzadeh, I.; Teimuri-Mofrad, R. Synthesis, characterization and electrochemical properties of 4-azidobutylferrocene-grafted reduced graphene oxide-polyaniline nanocomposite for supercapacitor applications. ChemistrySelect 2020, 5, 575–583. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Payami, E.; Ahadzadeh, I. Synthesis, characterization and electrochemical evaluation of a novel high performance GO-Fc/PANI nanocomposite for supercapacitor applications. Electrochim. Acta 2019, 321, 134706. [Google Scholar] [CrossRef]
- Payami, E.; Ahadzadeh, I.; Mohammadi, R.; Teimuri-Mofrad, R. Design and synthesis of novel binuclear ferrocenyl-intercalated graphene oxide and polyaniline nanocomposite for supercapacitor applications. Electrochim. Acta 2020, 342, 136078. [Google Scholar] [CrossRef]
- Aceta, Y.; Leroux, Y.R.; Hapiot, P. Evaluation of alkyl-ferrocene monolayers on carbons for charge storage applications, a voltammetry and impedance spectroscopy investigation. ChemElectroChem 2019, 6, 1704–1710. [Google Scholar] [CrossRef]
- Qiu, Y.; Wang, X.; Huang, H.; Cheng, Z.; Chang, X.; Guo, L. Ferrocenyl-functionalized carbon nanotubes with greatly improved surface reactivity for enhancing electrocapacitance. J. Organomet. Chem. 2019, 880, 349–354. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Abbasi, H.; Payami, E.; NeshadGreen, S. Synthesis of carbon nanotubes@tetraferrocenylporphyrin/copper nanohybrid and evaluation of its ability as a supercapacitor. J. Organomet. Chem. 2019, 899, 120915. [Google Scholar] [CrossRef]
- Payami, E.; Aghaiepour, A.; Rahimpour, K.; Mohammadi, R.; Teimuri-Mofrad, R. Design and synthesis of ternary GO-Fc/Mn3O4/PANI nanocomposite for energy storage applications. J. Alloys Compd. 2020, 829, 154485. [Google Scholar] [CrossRef]
- Borenstein, A.; Strauss, V.; Kowal, M.D.; Yoonessi, M.; Muni, M.; Anderson, M.; Kaner, R.B. Laser-reduced graphene-oxide/ferrocene: A 3-D redox-active composite for supercapacitor electrodes. J. Mater. Chem. A 2018, 6, 20463–20472. [Google Scholar] [CrossRef]
- Madec, L.; Bouvre, A.; Blanchard, P.; Cougnon, C.; Brousse, T.; Lestriez, B.; Guyomarda, D.; Gaubicher, J. In situ redox functionalization of composite electrodes for high power-high energy electrochemical storage systems via a non-covalent approach Energy. Environ. Sci. 2012, 5, 5379–5386. [Google Scholar] [CrossRef]
- Teimuri-Mofrad, R.; Hadi, R.; Abbasi, H.; Baj, R.F.B. Synthesis, characterization and electrochemical study of carbon nanotube/chitosan-ferrocene nanocomposite electrode as supercapacitor material. J. Electron. Mater. 2019, 48, 4573–4581. [Google Scholar] [CrossRef]
- Yang, X.; Wang, Y.; Qi, W.; Zhang, J.; Zhang, L.; Huang, R.; Su, R.; He, Z. Photo-induced polymerization and reconfigurable assembly of multifunctional ferrocene-tyrosine. Small 2018, 14, 1800772. [Google Scholar] [CrossRef]
- Beasley, C.A.; Murray, R.W. Voltammetry and redox charge storage capacity of ferrocene-functionalized silica nanoparticles. Langmuir 2009, 25, 10370–10375. [Google Scholar] [CrossRef]
- Holze, R. From current peaks to waves and capacitive currents—On the origins of capacitor-like electrode behavior. J. Solid State Electrochem. 2017, 21, 2601–2607. [Google Scholar] [CrossRef]
- Rajak, R.; Saraf, M.; Mohammad, A.; Mobin, S.M. Design and construction of a ferrocene based inclined polycatenated Co-MOF for supercapacitor and dye adsorption applications. J. Mater. Chem. A 2017, 5, 17998–18011. [Google Scholar] [CrossRef]
- Lei, Z.B.; Chen, Z.W.; Zhao, X.S. Growth of polyaniline on hollow carbon spheres for enhancing electrocapacitance. J. Phys. Chem. C 2010, 114, 19867–19874. [Google Scholar] [CrossRef]
- Mao, X.; Simeon, F.; Achilleos, D.S.; Rutledge, G.C.; Hatton, T.A. Metallocene/carbon hybrids prepared by a solution process for supercapacitor applications. J. Mater. Chem. A 2013, 1, 13120–13127. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Malveau, C.; Rochefort, D. Solid-state NMR and electrochemical dilatometry study of charge storage in supercapacitor with redox ionic liquid electrolyte. Energy Storage Mater. 2019, 20, 80–88. [Google Scholar] [CrossRef]
- Xie, H.J.; Glinas, B.; Rochefort, D. Redox-active electrolyte supercapacitors using electroactive ionic liquids. Electrochem. Commun. 2016, 66, 42–45. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, Y.; Zhang, D.; Ma, J.; Yi, W.; Zhang, J.; Shi, H. Synthesis of multiwall carbon nanotubes via an inert atmosphere absent autogenetic-pressure method for supercapacitor. J. Energy Storage 2019, 26, 100995. [Google Scholar] [CrossRef]
















© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Holze, R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes—An Introduction. Polymers 2020, 12, 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081835
Holze R. Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes—An Introduction. Polymers. 2020; 12(8):1835. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081835
Chicago/Turabian StyleHolze, Rudolf. 2020. "Composites and Copolymers Containing Redox-Active Molecules and Intrinsically Conducting Polymers as Active Masses for Supercapacitor Electrodes—An Introduction" Polymers 12, no. 8: 1835. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081835