Synthesis and Properties of Moisture-Cured Reactive Polyurethane Containing Castor Oil and Oxime Compounds
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
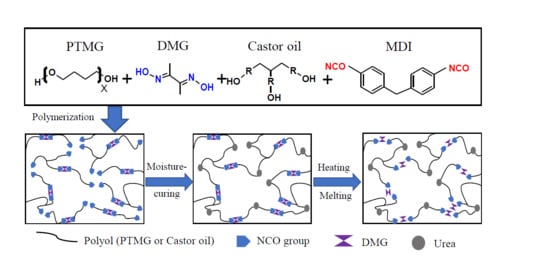
2.2. Synthesis of Moisture-Cured Reactive Polyurethane
2.3. Preparation of Cured Reactive Polyurethane Film
2.4. Properties of Moisture-Cured Reactive Polyurethane
2.5. FTIR Analysis
2.6. DSC Thermo-Analysis
2.7. DMA Thermo-Analysis
2.8. TGA Thermo-Analysis
2.9. Tensile Tests of Cured PUR Films
3. Results and Discussion
3.1. Properties of PUR
3.2. FTIR of PUR
3.3. Thermal Behavior of Cured PUR
3.4. TGA Thermo-Analysis of Cured PUR
3.5. Mechanical Property of Cured PUR
4. Conclusions
Funding
Conflicts of Interest
References
- Chattopadhyay, D.K.; Sreedhar, B.; Raju, K.V.S.N. Thermal stability of chemically crosslinked moisture-cured polyurethane coatings. J. Appl. Polym. Sci. 2005, 95, 1509–1518. [Google Scholar] [CrossRef]
- Sun, L.; Cao, S.; Xue, W.; Zeng, Z.; Zhu, W. Reactive hot melt polyurethane adhesives modified with pentaerythritol diacrylate: Synthesis and properties. J. Adhes. Sci. Technol. 2016, 30, 1212–1222. [Google Scholar] [CrossRef]
- Liu, W.X.; Zhang, C.; Zhang, H.; Zhao, N.; Yu, Z.X.; Xu, J. Oxime-based and catalyst-free dynamic covalent polyurethanes. J. Am. Chem. Soc. 2017, 139, 8678–8684. [Google Scholar] [CrossRef] [PubMed]
- Liang, H.Y.; Wang, S.W.; He, H.; Wang, M.Q.; Liu, L.X.; Lu, J.Y.; Zhang, C.Q. Aqueous anionic polyurethane dispersions from castor oil. Ind. Crops. Prod. 2018, 122, 182–189. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Y.; Liang, H.; Zhou, X.; Fang, C.; Zhang, C.; Luo, Y. Synthesis and properties of castor oil-based waterborne polyurethane/sodium alginate composites with tunable properties. Carbohydr. Polym. 2019, 208, 391–397. [Google Scholar] [CrossRef] [PubMed]
- Kim, A.R.; Park, M.S.; Lee, S.; Ra, M.; Shin, J.; Kim, Y.W. Polybutylene terephthalate modified with dimer acid methyl ester derived from fatty acid methyl esters and its use as a hot-melt adhesive. J. Appl. Polym. Sci. 2020, 137, 48474–48483. [Google Scholar] [CrossRef]
- Chung, C.H.; Shih, W.C.; Chiu, W.M. Synthesis, characterization and properties of biomass and carbon dioxide derived polyurethane reactive hot-melt adhesives. e-Polymers 2019, 19, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.C.; Tai, W. Castor oil-based polyurethane resin for low-density composites with bamboo charcoal. Polymers 2018, 10, 1100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawpan, M.A. Polyurethanes from vegetable oils and applications: A review. J. Polym. Res. 2018, 25, 184–199. [Google Scholar] [CrossRef]
- Gama, N.; Ferreira, A.; Barros-Timmons, A. Cure and performance of castor oil polyurethane adhesive. Int. J. Adhes. Adhes. 2019, 95, 102413–102420. [Google Scholar] [CrossRef]
- Daemi, H.; Barikani, M.; Barmar, M. Compatible compositions based on aqueous polyurethane dispersions and sodium alginate. Carbohydr. Polym. 2013, 92, 490–496. [Google Scholar] [CrossRef] [PubMed]
- Liang, D.; Zhang, Q.; Zhang, W.; Liu, L.; Liang, H.; Quirino, R.L.; Chen, J.; Liu, M.; Lu, Q.; Zhang, C. Tunable thermo-physical performance of castor oil-based polyurethanes with tailored release of coated fertilizers. J. Clean. Prod. 2019, 210, 1207–1215. [Google Scholar] [CrossRef]
- Pelufo, D.I.; Neto, S.C.; Gobbo, R.C.B.; Santos, A.J.; Terezo, A.J.; Siqueira, A.B. Kinetic study of the thermal decomposition of castor oil based polyurethane. J. Polym. Res. 2020, 27, 143–150. [Google Scholar] [CrossRef]
- Ravey, M.; Pearce, E.M. Flexible polyurethane foam. 1. Thermal decomposition of a polyether-based, water-blown commercial type of flexible polyurethane foam. J. Appl. Polym. Sci. 1997, 63, 47–74. [Google Scholar] [CrossRef]
- Krämer, R.H.; Zammarano, M.; Linteris, G.T.; Gedde, U.W..; Gilman, J.W. Heat release and structure collapse of flexible polyurethane foam. Polym. Degrad. Stabil. 2010, 95, 1115–1122. [Google Scholar] [CrossRef]
- Liang, H.Y.; Liu, L.X.; Lu, J.Y.; Chen, M.T.; Zhang, C.Q. Castor oil-based cationic waterborne polyurethane dispersions: Storage stability, thermo-physical properties and antibacterial properties. Ind. Crops. Prod. 2018, 117, 169–178. [Google Scholar] [CrossRef]
- Wang, C.; Zheng, Y.; Xie, Y.; Qiao, K.; Sun, Y.; Yue, L. Synthesis of bio-castor oil polyurethane flexible foams and the influence of biotic component on their performance. J. Polym. Res. 2015, 22, 145–154. [Google Scholar] [CrossRef]









| Code of Resins | First-Step Synthesis | Second-Step Synthesis | NCO% | Viscosity (ps/100 °C) | |||
|---|---|---|---|---|---|---|---|
| PTMG 1 (g) | Castor oil (g) | MDI 1,2 (g) | DMG 1,3 (wt%) | DMG 1,3 (wt%) | |||
| PUR | 100 | 20 | 63 | - | - | 5.73 | 71 |
| PUR-I-1.5 | 100 | 20 | 63 | 1.5 | - | 4.60 | 150 |
| PUR-I-3.0 | 100 | 20 | 63 | 3.0 | - | 3.48 | 252 |
| PUR-II-1.5 | 100 | 20 | 63 | - | 1.5 | 4.56 | 181 |
| PUR-II-3.0 | 100 | 20 | 63 | - | 3.0 | 3.41 | 320 |
| Code of Resins | TGA Parameters | Mechanical Properties | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| I Onset Temp. (°C) | I Weight Loss (%) | II Onset Temp. (°C) | II Weight Loss (%) | III Onset Temp. (°C) | III Weight Loss (%) | 650 °C Residues (%) | Tensile Strength (MPa) | Young’s Modulus (MPa) | Elongation (%) | |
| PUR | - | - | 318.7 | 32.1 | 435.9 | 62.5 | 5.0 | 22.1 | 8.8 | 108.5 |
| PUR-I-1.5 | 186.1 | 1.8 | 312.7 | 31.6 | 426.1 | 63.2 | 5.1 | 23.1 | 8.8 | 100.1 |
| PUR-I-3.0 | 195.2 | 4.1 | 315.5 | 30.1 | 418.5 | 59.3 | 6.5 | 25.1 | 8.9 | 77.2 |
| PUR-II-1.5 | 188.5 | 1.3 | 317.2 | 31.1 | 434.1 | 61.2 | 4.6 | 20.1 | 7.8 | 96.1 |
| PUR-II-3.0 | 191.5 | 3.5 | 319.2 | 30.5 | 435.2 | 62.1 | 4.9 | 20.3 | 7.9 | 71.4 |
© 2020 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Z.-Y. Synthesis and Properties of Moisture-Cured Reactive Polyurethane Containing Castor Oil and Oxime Compounds. Polymers 2020, 12, 1838. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081838
Wu Z-Y. Synthesis and Properties of Moisture-Cured Reactive Polyurethane Containing Castor Oil and Oxime Compounds. Polymers. 2020; 12(8):1838. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081838
Chicago/Turabian StyleWu, Zheng-Ying. 2020. "Synthesis and Properties of Moisture-Cured Reactive Polyurethane Containing Castor Oil and Oxime Compounds" Polymers 12, no. 8: 1838. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12081838