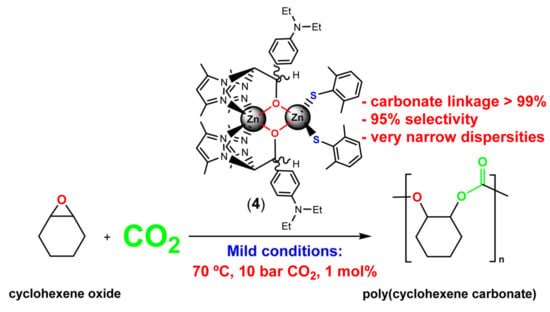
Efficient Production of Poly(Cyclohexene Carbonate) via ROCOP of Cyclohexene Oxide and CO2 Mediated by NNO-Scorpionate Zinc Complexes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Experimental
2.2.1. Nuclear Magnetic Resonance Spectroscopy (NMR)
2.2.2. Elemental Analysis
2.2.3. Gel Permeation Chromatography (GPC)
2.2.4. Crystallographic Refinement and Structure Solution
2.3. General Procedures
2.3.1. Preparation of Compounds 1−6
2.3.2. General Procedure for the Synthesis of Polycarbonates
3. Results
3.1. Synthesis and Characterization of Complexes
3.2. Catalytic Studies on the Ring-Opening Copolymerization of Cyclohexene Oxide with Carbon Dioxide
Polymer Microstructure and End-Group Analysis of Poly(Cyclohexene Carbonate) Produced by Complex 4
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Grignard, B.; Gennen, S.; Jérôme, C.; Kleij, A.W.; Detrembleur, C. Advances in the use of CO2 as a renewable feedstock for the synthesis of polymers. Chem. Soc. Rev. 2019, 48, 4466–4514. [Google Scholar] [CrossRef]
- Artz, J.; Müller, T.E.; Thenert, K.; Kleinekorte, J.; Meys, R.; Sternberg, A.; Bardow, A.; Leitner, W. Sustainable conversion of carbon dioxide: An integrated review of catalysis and life cycle assessment. Chem. Rev. 2018, 118, 434–504. [Google Scholar] [CrossRef]
- Dabral, S.; Schaub, T. The use of carbon dioxide (CO2) as a building block in organic synthesis from an industrial perspective. Adv. Synth. Catal. 2019, 361, 223–246. [Google Scholar] [CrossRef] [Green Version]
- Poland, S.J.; Darensbourg, D.J. A quest for polycarbonates provided via sustainable epoxide/CO2 copolymerization processes. Green Chem. 2017, 19, 4990–5011. [Google Scholar] [CrossRef]
- Zhu, Y.; Romain, C.; Williams, C.K. Sustainable polymers from renewable resources. Nature 2016, 540, 354–362. [Google Scholar] [CrossRef]
- Guo, W.; Gómez, J.E.; Cristòfol, À.; Xie, J.; Kleij, A.W. Catalytic transformations of functionalized cyclic organic carbonates. Angew. Chem. Int. Ed. 2018, 57, 13735–13747. [Google Scholar] [CrossRef]
- Huang, J.; Worch, J.C.; Dove, A.P.; Coulembier, O. Update and challenges in carbon dioxide-based polycarbonate synthesis. ChemSusChem 2019, 13, 469–487. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Lin, L.; Xiao, M.; Wang, S.; Smith, A.T.; Sun, L.; Meng, Y. Synthesis and properties of CO2-based plastics: Environmentally-friendly, energy-saving and biomedical polymeric materials. Prog. Polym. Sci. 2018, 80, 163–182. [Google Scholar] [CrossRef]
- Paul, S.; Romain, C.; Shaw, J.; Williams, C.K. Sequence selective polymerization catalysis: A new route to aba block copoly(ester-b-carbonate-b-ester). Macromolecules 2015, 48, 6047–6056. [Google Scholar] [CrossRef] [Green Version]
- Helou, M.; Carpentier, J.-F.; Guillaume, S.M. Poly(carbonate-urethane): An isocyanate-free procedure from α,ω-di(cyclic carbonate) telechelic poly(trimethylene carbonate)s. Green Chem. 2011, 13, 266–271. [Google Scholar] [CrossRef]
- Paul, S.; Zhu, Y.; Romain, C.; Brooks, R.; Saini, P.K.; Williams, C.K. Ring-opening copolymerization (ROCOP): Synthesis and properties of polyesters and polycarbonates. Chem. Commun. 2015, 51, 6459–6479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sulley, G.S.; Gregory, G.L.; Chen, T.T.D.; Peña-Carrodeguas, L.; Trott, G.; Santmarti, A.; Lee, K.; Terrill, N.J.; Williams, C.K. Switchable catalysis improves the properties of CO2-derived polymers: Poly(cyclohexene carbonate-b-ε-decalactone-b-cyclohexene carbonate) adhesives, elastomers, and toughened plastics. J. Am. Chem. Soc. 2020, 142, 4367–4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, Q.-W.; Zhou, Z.-H.; He, L.-N. Efficient, selective and sustainable catalysis of carbon dioxide. Green Chem. 2017, 19, 3707–3728. [Google Scholar] [CrossRef]
- Pescarmona, P.P.; Taherimehr, M. Challenges in the catalytic synthesis of cyclic and polymeric carbonates from epoxides and CO2. Catal. Sci. Technol. 2012, 2, 2169–2187. [Google Scholar] [CrossRef]
- Andrea, K.A.; Kerton, F.M. Triarylborane-catalyzed formation of cyclic organic carbonates and polycarbonates. ACS Catal. 2019, 9, 1799–1809. [Google Scholar] [CrossRef]
- Trott, G.; Saini, P.K.; Williams, C.K. Catalysts for CO2/epoxide ring-opening copolymerization. Philos. Trans. R. Soc. A Math. Phys Eng. Sci. 2016, 374, 20150085. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pankhurst, J.R.; Paul, S.; Zhu, Y.; Williams, C.K.; Love, J.B. Polynuclear alkoxy–zinc complexes of bowl-shaped macrocycles and their use in the copolymerisation of cyclohexene oxide and CO2. Dalton Trans. 2019, 48, 4887–4893. [Google Scholar] [CrossRef] [Green Version]
- Reiter, M.; Vagin, S.; Kronast, A.; Jandl, C.; Rieger, B. A Lewis acid β-diiminato-zinc-complex as all-rounder for co- and terpolymerisation of various epoxides with carbon dioxide. Chem. Sci. 2017, 8, 1876–1882. [Google Scholar] [CrossRef] [Green Version]
- Kernbichl, S.; Reiter, M.; Adams, F.; Vagin, S.; Rieger, B. CO2-Controlled One-Pot Synthesis of AB, ABA Block, and Statistical Terpolymers from β-Butyrolactone, Epoxides, and CO2. J. Am. Chem. Soc. 2017, 139, 6787–6790. [Google Scholar] [CrossRef]
- Schütze, M.; Dechert, S.; Meyer, F. Highly Active and Readily Accessible Proline-Based Dizinc Catalyst for CO2/Epoxide Copolymerization. Chem. Eur. J. 2017, 23, 16472–16475. [Google Scholar] [CrossRef]
- Thevenon, A.; Garden, J.A.; White, A.J.P.; Williams, C.K. Dinuclear Zinc Salen Catalysts for the Ring Opening Copolymerization of Epoxides and Carbon Dioxide or Anhydrides. Inorg. Chem. 2015, 54, 11906–11915. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissling, S.; Lehenmeier, M.W.; Altenbuchner, P.T.; Kronast, A.; Reiter, M.; Deglmann, P.; Seemann, U.B.; Rieger, B. Dinuclear zinc catalysts with unprecedented activities for the copolymerization of cyclohexene oxide and CO2. Chem. Commun. 2015, 51, 4579–4582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kissling, S.; Altenbuchner, P.T.; Lehenmeier, M.W.; Herdtweck, E.; Deglmann, P.; Seemann, U.B.; Rieger, B. Mechanistic aspects of a highly active dinuclear zinc catalyst for the co-polymerization of epoxides and CO2. Chem. Eur. J. 2015, 21, 8148–8157. [Google Scholar] [CrossRef] [PubMed]
- Kember, M.R.; Knight, P.D.; Reung, P.T.R.; Williams, C.K. Highly active dizinc catalyst for the copolymerization of carbon dioxide and cyclohexene oxide at one atmosphere pressure. Angew. Chem. Int. Ed. 2009, 48, 931–933. [Google Scholar] [CrossRef]
- Moore, D.R.; Cheng, M.; Lobkovsky, E.B.; Coates, G.W. Electronic and steric effects on catalysts for CO2/epoxide polymerization: Subtle modifications resulting in superior activities. Angew. Chem. Int. Ed. 2002, 41, 2599–2602. [Google Scholar] [CrossRef]
- Ni, K.; Kozak, C.M. Kinetic studies of copolymerization of cyclohexene oxide with CO2 by a diamino-bis(phenolate) chromium(iii) complex. Inorg. Chem. 2018, 57, 3097–3106. [Google Scholar] [CrossRef]
- Castro-Osma, J.A.; Lamb, K.J.; North, M. Cr(salophen) complex catalyzed cyclic carbonate synthesis at ambient temperature and pressure. ACS Catalyst 2016, 6, 5012–5025. [Google Scholar] [CrossRef]
- Viciano, M.; Munoz, B.K.; Godard, C.; Castillon, S.; Reyes, M.L.; Garcia-Ruiz, M.; Claver, C. Salcy-naphthalene cobalt complexes as catalysts for the synthesis of high molecular weight polycarbonates. ChemCatChem 2017, 9, 3974–3981. [Google Scholar] [CrossRef]
- Hatazawa, M.; Nakabayashi, K.; Ohkoshi, S.; Nozaki, K. In situ generation of CoIII–Salen complexes for copolymerization of propylene oxide and CO2. Chem. Eur. J. 2016, 22, 13677–13681. [Google Scholar] [CrossRef]
- Andrea, K.A.; Butler, E.D.; Brown, T.R.; Anderson, T.S.; Jagota, D.; Rose, C.; Lee, E.M.; Goulding, S.D.; Murphy, J.N.; Kerton, F.M.; et al. Iron complexes for cyclic carbonate and polycarbonate formation: Selectivity control from ligand design and metal-center geometry. Inorg. Chem. 2019, 58, 11231–11240. [Google Scholar] [CrossRef]
- Gu, G.-G.; Yue, T.-J.; Wan, Z.-Q.; Zhang, R.; Lu, X.-B.; Ren, W.-M. A Single-site iron(III)-salan catalyst for converting COS to sulfur-containing polymers. Polymers 2017, 9, 515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hua, L.; Li, B.; Han, C.; Gao, P.; Wang, Y.; Yuan, D.; Yao, Y. Synthesis of homo- and heteronuclear rare-earth metal complexes stabilized by ethanolamine-bridged bis(phenolato) ligands and their application in catalyzing reactions of CO2 and epoxides. Inorg. Chem. 2019, 58, 8775–8786. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Xu, C.; Han, Q.; Tang, X.; Zhou, P.; Zhang, R.; Gao, G.; Xu, B.; Qin, W.; Liu, W. Ambient chemical fixation of CO2 using a highly efficient heterometallic helicate catalyst system. Chem. Commun. 2018, 54, 2212–2215. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, Y.J.; Ye, Y.S.; Peng, H.Y.; Zhou, X.P.; Xie, X.L.; Wang, X.H.; Wang, F.S. A one-step route to CO2-based block copolymers by simultaneous ROCOP of CO2/epoxides and RAFT polymerization of vinyl monomers. Angew. Chem. Int. Ed. 2018, 57, 3593–3597. [Google Scholar] [CrossRef] [PubMed]
- Kindermann, N.; Cristofol, A.; Kleij, A.W. Access to biorenewable polycarbonates with unusual glass-transition temperature (Tg) modulation. ACS Catal. 2017, 7, 3860–3863. [Google Scholar] [CrossRef]
- Shaikh, R.R.; Pornpraprom, S.; D’Elia, V. Catalytic strategies for the cycloaddition of pure, diluted, and waste CO2 to epoxides under ambient conditions. ACS Catalyst 2018, 8, 419–450. [Google Scholar] [CrossRef]
- Deacy, A.C.; Kilpatrick, A.F.R.; Regoutz, A.; Williams, C.K. Understanding metal synergy in heterodinuclear catalysts for the copolymerization of CO2 and epoxides. Nat. Chem. 2020, 12, 372–380. [Google Scholar] [CrossRef]
- Trott, G.; Garden, J.A.; Williams, C.K. Heterodinuclear zinc and magnesium catalysts for epoxide/CO2 ring opening copolymerizations. Chem. Sci. 2019, 10, 4618–4627. [Google Scholar] [CrossRef] [Green Version]
- July: Carbon Dioxide. Available online: http://www.novomer.com/?action=CO2 (accessed on 21 July 2020).
- Empower Materials. Available online: http://www.empowermaterials.com (accessed on 21 July 2020).
- Ok, M.-A.; Jeon, M. Properties of poly(propylene carbonate) produced via SK Energy’s Greenpol™ Technology, ANTEC 2011, Society of Plastics Engineers, Econic Technologies. Available online: http://www.econic-technologies.com/ (accessed on 10 July 2020).
- Kannan, M.B.; Moore, C.; Saptarshi, S.; Somasundaram, S.; Rahuma, M.; Lopata, A.L. Biocompatibility and biodegradation studies of a commercial zinc alloy for temporary mini-implant applications. Sci. Rep. 2017, 7, 15605. [Google Scholar] [CrossRef] [Green Version]
- Levy, G.K.; Goldman, J.; Aghion, E. The Prospects of Zinc as a Structural Material for Biodegradable Implants—A Review Paper. Metals 2017, 7, 402. [Google Scholar] [CrossRef] [Green Version]
- Nakanishi, T. Potential toxicity of organotin compounds via nuclear receptor signaling in mammals. J. Health Sci. 2007, 53, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Appel, K.E. Organotin Compounds: Toxicokinetic Aspects. Drug Metab. Rev. 2004, 36, 763–786. [Google Scholar] [CrossRef] [PubMed]
- Otero, A.; Fernández-Baeza, J.; Lara-Sánchez, A.; Sánchez-Barba, L.F. Metal complexes with heteroscorpionate ligands based on the bis(pyrazol-1-yl)methane moiety: Catalytic chemistry. Coord. Chem. Rev. 2013, 257, 1806–1868. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Copolymerization of cyclic esters controlled by chiral NNO-scorpionate zinc initiators. Organometallics 2016, 35, 189–197. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Synthesis and dynamic behavior of chiral NNO-scorpionate zinc initiators for the ring-opening polymerization of cyclic esters. Eur. J. Inorg. Chem. 2016, 2562–2572. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Martínez-Ferrer, J.; Sobrino, S.; Rodríguez, A.M. New racemic and single enantiopure hybrid scorpionate/cyclopentadienyl magnesium and zinc initiators for the stereoselective ROP of lactides. Organometallics 2015, 34, 3196–3208. [Google Scholar] [CrossRef]
- Honrado, M.; Otero, A.; Fernandez-Baeza, J.; Sanchez-Barba, L.F.; Garcés, A.; Lara-Sanchez, A.; Rodriguez, A.M. Enantiopure N,N,O-scorpionate zinc amide and chloride complexes as efficient initiators for the heteroselective ROP of cyclic esters. Dalton Trans. 2014, 43, 17090–17100. [Google Scholar] [CrossRef]
- Martínez, J.; Castro-Osma, J.A.; Lara-Sánchez, A.; Otero, A.; Fernández-Baeza, J.; Tejeda, J.; Sánchez-Barba, L.F.; Rodríguez-Diéguez, A. Ring-opening copolymerisation of cyclohexene oxide and carbon dioxide catalysed by scorpionate zinc complexes. Polym. Chem. 2016, 7, 6475–6484. [Google Scholar] [CrossRef]
- Sobrino, S.; Navarro, M.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Garcés, A.; Lara-Sánchez, A.; Castro-Osma, J.A. Efficient CO2 fixation into cyclic carbonates catalyzed by NNO-scorpionate zinc complexes. Dalton Trans. 2019, 48, 10733–10742. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Sobrino, S.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Mono- and binuclear chiral N,N,O-scorpionate zinc alkyls as efficient initiators for the ROP of rac-lactide. Dalton Trans. 2017, 46, 15107–15117. [Google Scholar] [CrossRef]
- SAINT v8.37, Bruker-AXS, APEX3 v2016.1.0; Bruker: Madison, WI, USA, 2016.
- Krause, L.; Herbst-Irmer, R.; Sheldrick, G.M.; Stalke, D. Comparison of silver and molybdenum microfocus X-ray sources for single-crystal structure determination. J. Appl. Crystallogr. 2015, 48, 3–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farrugia, L.J. WinGX and ORTEP for Windows: An update. J. Appl. Cryst. 2012, 45, 849–854. [Google Scholar] [CrossRef]
- Sheldrick, G.M. SHELX-2014, Program for Crystal Structure Refinement; University of Göttingen: Göttingen, Germany, 2014. [Google Scholar]
- Sluis, P.V.D.; Spek, A.L. BYPASS: An effective method for the refinement of crystal structures containing disordered solvent regions. Acta Crystallogr. Sect. A Found. Crystallogr. 1990, 46, 194–201. [Google Scholar] [CrossRef]
- Timothy, J.B.; Pratt, H.D., III; Alam, T.M.; Headley, T.; Rodriguez, M.A. Synthesis and characterization of thiolate-oxo ligated zinc alkyl derivatives for production of Zn-based nanoparticles. Eur. J. Inorg. Chem. 2009, 855–865. [Google Scholar] [CrossRef] [Green Version]
- Grunova, E.; Roisnel, T.; Carpentier, J.-F. Zinc complexes of fluorous alkoxide-iminoligands: Synthesis, structure, and use in ring-opening polymerization of lactide and β-butyrolactone. Dalton Trans. 2009, 9010–9019. [Google Scholar] [CrossRef]
- Poirier, V.; Roisnel, T.; Carpentier, J.-F.; Sarazin, Y. Versatile catalytic systems based on complexes of zinc, magnesium and calcium supported by a bulky bis(morpholinomethyl)phenoxy ligand for the large-scale immortal ring-opening polymerisation of cyclic esters. Dalton Trans. 2009, 9820–9827. [Google Scholar] [CrossRef]
- Johnson, A.L.; Hollingsworth, N.; Kociok-Köhn, G.; Molloy, K.C. Organozinc aminoalcoholates: Synthesis, structure, and materials chemistry. Inorg. Chem. 2008, 47, 12040–12048. [Google Scholar] [CrossRef]
- Otero, A.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Tejeda, J.; Honrado, M.; Garcés, A.; Lara-Sánchez, A.; Rodríguez, A.M. Chiral N,N,O-scorpionate zinc alkyls as effective and stereoselective initiators for the living ROP of lactides. Organometallics 2012, 31, 4191–4202. [Google Scholar] [CrossRef]
- Alonso-Moreno, C.; Garcés, A.; Sánchez-Barba, L.F.; Fajardo, M.; Fernández-Baeza, J.; Otero, A.; Antiñolo, A.; Lara-Sánchez, A.; Broomfield, L.; López-Solera, I.; et al. Discrete heteroscorpionate lithium and zinc alkyl complexes. synthesis, structural studies, and ROP of cyclic esters. Organometallics 2008, 27, 1310–1321. [Google Scholar] [CrossRef]
- Paradiso, V.; Capaccio, V.; Lamparelli, D.H.; Capacchione, C. Metal complexes bearing sulfur-containing ligands as catalysts in the reaction of CO2 with epoxides. Catalysts 2020, 10, 825. [Google Scholar] [CrossRef]
- Kember, M.R.; Williams, C.K. Efficient magnesium catalysts for the copolymerization of epoxides and CO2; using water to synthesize polycarbonate polyols. J. Am. Chem. Soc. 2012, 134, 15676–15679. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Wang, Z.; Ding, K. Intramolecularly dinuclear magnesium complex catalyzed copolymerization of cyclohexene oxide with CO2 under ambient CO2 Pressure: Kinetics and mechanism. Macromolecules 2006, 39, 128–137. [Google Scholar] [CrossRef]








| Bond Lengths | |||||
| 4 | 5 | 6 | |||
| N(1)-Zn(1) | 2.133(7) | Zn(1)-N(1) | 2.047(5) | Zn(1)-N(1) | 2.047(3) |
| N(3)-Zn(1) | 2.230(7) | Zn(1)-N(3) | 2.067(5) | Zn(1)-N(3) | 2.082(3) |
| N(6)-Zn(1) | 2.117(7) | Zn(1)-S(2) | 2.265(2) | Zn(1)-S(1) | 2.268(1) |
| N(8)-Zn(1) | 2.226(7) | Zn(1)-S(1) | 2.290(2) | Zn(1)-S(2) | 2.296(1) |
| O(1)-Zn(2) | 2.032(5) | S(1)-C(29) | 1.763(8) | S(1)-C(23) | 1.769(5) |
| O(1)-Zn(1) | 2.044(5) | S(2)-C(21) | 1.765(7) | S(2)-C(31) | 1.771(5) |
| O(2)-Zn(2) | 2.036(4) | O(1)-C(12) | 1.414(7) | O(1)-C(12) | 1.414(4) |
| O(2)-Zn(1) | 2.048(5) | ||||
| S(1)-Zn(2) | 2.287(3) | ||||
| S(2)-Zn(2) | 2.277(3) | ||||
| Zn(1)-Zn(2) | 2.974(1) | ||||
| C(45)-S(1) | 1.789(9) | ||||
| C(53)-S(2) | 1.763(9) | ||||
| Angles | |||||
| S(2)-Zn(2)-S(1) | 134.15(9) | S(2)-Zn(1)-S(1) | 99.87(8) | S(1)-Zn(1)-S(2) | 98.87(4) |
| O(1)-Zn(1)-O(2) | 86.1(2) | N(1)-Zn(1)-N(3) | 91.6(2) | N(1)-Zn(1)-N(3) | 91.7(1) |
| N(6)-Zn(1)-N(1) | 98.7(3) | N(1)-Zn(1)-S(1) | 115.1(2) | N(1)-Zn(1)-S(1) | 122.9(1) |
| N(8)-Zn(1)-N(3) | 176.9(3) | N(3)-Zn(1)-S(2) | 114.3(2) | N(3)-Zn(1)-S(2) | 113.6(1) |
| O(1)-Zn(2)-O(2) | 86.7(2) | ||||
| Entry | Cat | Conv. (%) b | %CHC b | %Copolymer (%Carbonate Linkage) b | TON c | TOF d (h−1) |
|---|---|---|---|---|---|---|
| 1 | 2 | 52 | 44 | 56(64) | 29 | 1.82 |
| 2 | 4 | 75 | 7 | 93(>99) | 70 | 4.36 |
| 3 | 6 | 65 | 15 | 85(64) | 55 | 3.45 |
| Entry | Temp (°C) | Conv. (%) b | %CHC b | %Copolymer (%Carbonate Linkage) b | TON c | TOF d (h−1) | Mn(exp)(Da) e (Mw/Mn) e |
|---|---|---|---|---|---|---|---|
| 1 | 50 | 52 | 27 | 73(>99) | 38 | 2.37 | 5100(1.33) |
| 2 | 60 | 62 | 11 | 89(>99) | 55 | 3.45 | 7700(1.27) |
| 3 | 70 | 79 | 8 | 92(>99) | 73 | 4.54 | 10,700(1.03) |
| 4 | 80 | 75 | 7 | 93(>99) | 70 | 4.36 | 10,100(1.07) |
| 5 | 100 | 80 | 28 | 72(>99) | 58 | 3.60 | 8500(1.15) |
| Entry | Pres. (bar) | Conv. (%) b | %CHC b | %Copolymer (%Carbonate Linkage) b | TON c | TOF d (h−1) | Mn(exp)(Da) e (Mw/Mn) e |
|---|---|---|---|---|---|---|---|
| 1 | 1 | 55 | 10 | 90(>99) | 50 | 3.09 | 7400(1.13) |
| 2 | 10 | 72 | 5 | 95(>99) | 68 | 4.27 | 9500(1.09) |
| 3 | 20 | 70 | 3 | 97(>99) | 68 | 4.24 | 9800(1.03) |
| 4 | 30 | 83 | 17 | 83(>99) | 69 | 4.30 | 10,000(1.07) |
| 5 | 40 | 79 | 8 | 92(>99) | 73 | 4.54 | 10,700(1.03) |
| Entry | Time (h) | Conv. (%) b | %CHC b | %Copolymer (%Carbonate Linkage) b | TON c | TOF d (h−1) | Mn(exp)(Da) e (Mw/Mn) e |
|---|---|---|---|---|---|---|---|
| 1 | 2 | 17 | 16 | 84(>99) | 14 | 7.14 | 2400(1.33) |
| 2 | 5 | 32 | 14 | 86(>99) | 28 | 5.50 | 4500(1.11) |
| 3 | 8 | 49 | 9 | 91(>99) | 45 | 5.57 | 6600(1.14) |
| 4 | 10 | 61 | 12 | 88(>99) | 54 | 5.37 | 7900(1.13) |
| 5 | 15 | 70 | 7 | 93(>99) | 65 | 4.34 | 9400(1.05) |
| 6 | 16 | 72 | 5 | 95(>99) | 68 | 4.27 | 9500(1.09) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sobrino, S.; Navarro, M.; Fernández-Baeza, J.; Sánchez-Barba, L.F.; Lara-Sánchez, A.; Garcés, A.; Castro-Osma, J.A.; Rodríguez, A.M. Efficient Production of Poly(Cyclohexene Carbonate) via ROCOP of Cyclohexene Oxide and CO2 Mediated by NNO-Scorpionate Zinc Complexes. Polymers 2020, 12, 2148. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092148
Sobrino S, Navarro M, Fernández-Baeza J, Sánchez-Barba LF, Lara-Sánchez A, Garcés A, Castro-Osma JA, Rodríguez AM. Efficient Production of Poly(Cyclohexene Carbonate) via ROCOP of Cyclohexene Oxide and CO2 Mediated by NNO-Scorpionate Zinc Complexes. Polymers. 2020; 12(9):2148. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092148
Chicago/Turabian StyleSobrino, Sonia, Marta Navarro, Juan Fernández-Baeza, Luis F. Sánchez-Barba, Agustín Lara-Sánchez, Andrés Garcés, José A. Castro-Osma, and Ana M. Rodríguez. 2020. "Efficient Production of Poly(Cyclohexene Carbonate) via ROCOP of Cyclohexene Oxide and CO2 Mediated by NNO-Scorpionate Zinc Complexes" Polymers 12, no. 9: 2148. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092148