Raman Spectroscopy Study of Structurally Uniform Hydrogenated Oligomers of α-Olefins
Abstract
:1. Introduction
2. Materials and Methods
2.1. Sample Preparation
2.2. Raman Measurements
2.3. Quantum-Chemical Calculations
3. Results and Discussion
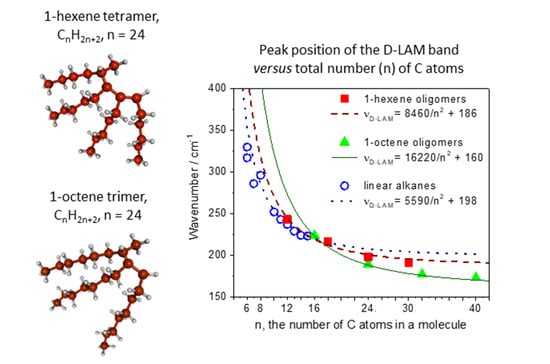
3.1. Spectral Region 60–500 cm−1
3.2. Symmetric C–C Stretching Mode
3.2.1. Spectral Studies
3.2.2. DFT Calculations
- the extended-chain conformation: RSA1139 = 17.27
- the branched 1 chain conformation: RSA1143 + RSA1149 = 14.59
- the branched 2 chain conformation: RSA1142 + RSA1150 = 14.98.
- the extended-chain conformation: RSA1136 = 27.87
- the branched 1 chain conformation: RSA1140 + RSA1146 = 21.40
- the branched 2 chain conformation: RSA1140 + RSA1147 = 22.40.
3.3. The Region 2750–3150 cm−1
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Linear Alkane | Symmetric C–C Stretching Mode Wavenumbers, cm−1 | LAM Wavenumbers, cm−1 | D-LAM Wavenumbers, cm−1 | |||
|---|---|---|---|---|---|---|
| Observed Value | DFT calc. Value | νC–C2 | Observed Value | DFT Calc. Value | Observed Value | |
| C5H12 | 1145 | 1142 | 1145 | 399 | 393 | – |
| C6H14 | 1142 | 1139 | 1141 | 370 | 362 | 317/330 |
| C7H16 | 1139 | 1137 | 1138 | 307 | 298 | 286 |
| C8H18 | 1137 | 1135 | 1137 | 280 | 270 | 296 |
| C9H20 | 1135 | 1134 | 1135 | 248 3 | 237 | 267 3 |
| C10H22 | 1134 | 1133 | 1134 | 230 | 219 | 252 |
| C11H24 | 1134 | 1133 | 1134 | 221 | 195 | 243 |
| C12H26 | 1134 | 1132 | 1133 | 212 | 184 | 237 |
| C13H28 | 1133 | 1132 | 1133 | 199 | 172 | 229 |
| C14H30 | 1133 | 1131 | 1133 | 171 | 159 | 224 |
| C15H32 | 1133 | 1131 | 1132 | 158 4 | 149 | 223 |
| C16H34 | 1133 | 1131 | 1132 | 150 5 | 139 | 220 7 |
| C17H36 | 1132 | 1130 | 1132 | 141 4 | 132 | 220 7 |
| C18H38 | 1130 | 1130 | 1132 | 133 6 | 124 | Lines are absent at room temperature |
| C20H42 | 1133 | 1130 | 1132 | 120 | 112 | |
References
- Nifant’ev, I.E.; Vinogradov, A.A.; Vinogradov, A.A.; Sedov, I.V.; Dorokhov, V.G.; Lyadov, A.S.; Ivchenko, P.V. Structurally uniform 1-hexene, 1-octene, and 1-decene oligomers: Zirconocene/MAO-catalyzed preparation, characterization, and prospects of their use as low-viscosity low-temperature oil base stocks. Appl. Catal. A Gen. 2018, 549, 40–50. [Google Scholar] [CrossRef]
- Jalali, A.; Nekoomanehs-Haghighi, M.; Dehghani, S.; Bahri-Laleh, N. Effect of metal type on the metallocene-catalyzed oligomerization of 1-hexene and 1-octene to produce polyα-olefin-based synthetic lubricants. Appl. Organomet. Chem. 2020, 34, e5338. [Google Scholar] [CrossRef]
- Nifant’ev, I.; Ivchenko, P. Fair Look at Coordination Oligomerization of Higher α-Olefins. Polymers 2020, 12, 1082. [Google Scholar] [CrossRef] [PubMed]
- Brown, K.G.; Bicknell-Brown, E.; Ladjadj, M. Raman-active bands sensitive to motion and conformation at the chain termini and backbones of alkanes and lipids. J. Phys. Chem. 1987, 91, 3436–3442. [Google Scholar] [CrossRef]
- Dmitriev, A.A.; Surovtsev, N.V. Temperature-Dependent Hydrocarbon Chain Disorder in Phosphatidylcholine Bilayers Studied by Raman Spectroscopy. J. Phys. Chem. B 2015, 119, 15613–15622. [Google Scholar] [CrossRef]
- Šebek, J.; Pele, L.; Potma, E.O.; Gerber, R.B. Raman spectra of long chain hydrocarbons: Anharmonic calculations, experiment and implications for imaging of biomembranes. Phys. Chem. Chem. Phys. 2011, 13, 12724–12733. [Google Scholar] [CrossRef] [Green Version]
- Sagitova, E.A.; Nikolaeva, G.Y.; Prokhorov, K.A.; Pashinin, P.P.; Ivchenko, P.V.; Vinogradov, A.A.; Nifant’ev, I.E. Raman spectroscopic identification of the chemical and conformational compositions of novel hydrogenated 1-hexene oligomers. Laser Phys. 2019, 29, 015701. [Google Scholar] [CrossRef]
- Pietralla, M.; Hotz, R.; Engst, T.; Siems, R. Chain direction elastic modulus of PE crystal and interlamellar force constant of n-alkane crystals from RAMAN measurements. J. Polym. Sci. Part B Polym. Phys. 1997, 35, 47–57. [Google Scholar] [CrossRef]
- Ikedou, K.; Yamamoto, H.; Nagashima, H.; Nemoto, N.; Tashiro, K. Crystal Structures of n-Alkanes with Branches of Different Size in the Middle. J. Phys. Chem. B 2005, 109, 10668–10675. [Google Scholar] [CrossRef]
- Yamamoto, H.; Nemoto, N.; Tashiro, K. Crystal Structure of 20-Methyl-Nonatriacontane ((C19H39)2CHCH3) and Its Compatibility with Nonatriacontane (C39H80). J. Phys. Chem. B 2004, 108, 5827–5835. [Google Scholar] [CrossRef]
- Yamamoto, H.; Tashiro, K.; Nemoto, N.; Motoyama, Y.; Takahashi, Y. Systematic Study of Aggregation Structure and Thermal Behavior of a Series of Unique H-Shape Alkane Molecules. J. Phys. Chem. B 2011, 115, 9537–9546. [Google Scholar] [CrossRef] [PubMed]
- Lewandowski, H.; Meier, R.J. On the longitudinal acoustic mode in branched and unsaturated polyethylenes. Macromol. Indian J. 2010, 6, 60–64. [Google Scholar]
- Soutzidou, M.; Masters, A.J.; Viras, K.; Booth, C. The LAM-1 band in the low-frequency Raman spectra of even and odd monosubstituted n-alkanes. Phys. Chem. Chem. Phys. 1999, 1, 415–419. [Google Scholar] [CrossRef]
- Amorim da Costa, A.M.; Geraldes, C.F.G.C.; Teixeira-Dias, J.J.C. A Raman spectroscopic study of molecular interaction in long-chain primary amines systems. J. Raman Spectrosc. 1982, 13, 56–62. [Google Scholar] [CrossRef]
- Snyder, R.G.; Kim, Y. Conformation and low-frequency isotropic Raman spectra of the liquid n-alkanes C4-C9. J. Phys. Chem. 1991, 95, 602–610. [Google Scholar] [CrossRef]
- Snyder, R.G. The structure of chain molecules in the liquid state: Low-frequency Raman spectra of n-alkanes and perfluoro-n-alkanes. J. Chem. Phys. 1982, 76, 3921–3927. [Google Scholar] [CrossRef]
- Brambilla, L.; Zerbi, G. Local Order in Liquid n-Alkanes: Evidence from Raman Spectroscopic Study. Macromolecules 2005, 38, 3327–3333. [Google Scholar] [CrossRef]
- Gorelik, V.S.; Verjaskin, S.S.; Sverbil, P.P.; Chervyakov, A.V.; Zlobina, L.I.; Sharts, O.N. Determination of the length of zigzag chain molecules from Raman spectra. Proc. SPIE 2001, 4469, 25–35. [Google Scholar] [CrossRef]
- Sagitova, E.A.; Donfack, P.; Prokhorov, K.A.; Nikolaeva, G.Y.; Gerasin, V.A.; Merekalova, N.D.; Materny, A.; Antipov, E.M.; Pashinin, P.P. Symmetric C–C Stretching Mode Splitting versus CH2-Chain Conformation Order in Sodium Montmorillonite Modified by Cetyltrimethylammonium Bromide. J. Phys. Chem. B 2012, 116, 221–231. [Google Scholar] [CrossRef]
- Rabolt, J.F.; Twieg, R.; Snyder, C. The influence of double bonds on the Raman active longitudinal acoustical model (LAM) in trans-alkenes. J. Chem. Phys. 1982, 76, 1646–1653. [Google Scholar] [CrossRef]
- Sagitova, E.A.; Donfack, P.; Prokhorov, K.A.; Nikolaeva, G.Y.; Gerasin, V.A.; Merekalova, N.D.; Materny, A.; Antipov, E.M.; Pashinin, P.P. Raman Spectroscopic Characterization of the Interlayer Structure of Na+-Montmorillonite Clay Modified by Ditetradecyl Dimethyl Ammonium Bromide. J. Phys. Chem. B 2009, 113, 7482–7490. [Google Scholar] [CrossRef] [PubMed]
- Ungar, G.; Zeng, X.; Brooke, G.M.; Mohammed, S. Structure and Formation of Noninteger and Integer Folded-Chain Crystals of Linear and Branched Monodisperse Ethylene Oligomers. Macromolecules 1998, 31, 1875–1879. [Google Scholar] [CrossRef]
- Ungar, G.; Zeng, X. Learning Polymer Crystallization with the Aid of Linear, Branched and Cyclic Model Compounds. Chem. Rev. 2001, 101, 4157–4188. [Google Scholar] [CrossRef] [PubMed]
- Shemouratov, Y.V.; Prokhorov, K.A.; Nikolaeva, G.Y.; Pashinin, P.P.; Kovalchuk, A.A.; Klyamkina, A.N.; Nedorezova, P.M.; Demidenok, K.V.; Lebedev, Y.A.; Antipov, E.M. Raman study of ethylene-propylene copolymers and polyethylene-polypropylene reactor blends. Laser Phys. 2008, 18, 554–557. [Google Scholar] [CrossRef]
- Kuznetsov, S.M.; Novikov, V.S.; Sagitova, E.A.; Ustynyuk, L.Y.; Glikin, A.A.; Prokhorov, K.A.; Nikolaeva, G.Y.; Pashinin, P.P. Raman spectra of n-pentane, n-hexane, and n-octadecane: Experimental and density functional theory (DFT) study. Laser Phys. 2019, 29, 085701. [Google Scholar] [CrossRef]
- Baker, J.; Pulay, P. Assessment of the Handy–Cohen optimized exchange density functional for organic reactions. J. Chem. Phys. 2002, 117, 1441–1449. [Google Scholar] [CrossRef]
- Laikov, D.N. Fast evaluation of density functional exchange-correlation terms using the expansion of the electron density in auxiliary basis sets. Chem. Phys. Lett. 1997, 281, 151–156. [Google Scholar] [CrossRef]
- Laikov, D.N. A new class of atomic basis functions for accurate electronic structure calculations of molecules. Chem. Phys. Lett. 2005, 416, 116–120. [Google Scholar] [CrossRef]
- Laikov, D.N.; Ustynyuk, Y.A. PRIRODA-04: A quantum-chemical program suite. New possibilities in the study of molecular systems with the application of parallel computing. Russ. Chem. Bull. 2005, 54, 820–826. [Google Scholar] [CrossRef]
- Parker, S.F.; Braden, D.A.; Tomkinson, J.; Hudson, B.S. Full Longitudinal Acoustic Mode (LAM) Spectrum of an N-Alkane: Comparison of Observed and Computed Incoherent Inelastic Neutron Scattering Spectrum of N-Octadecane. J. Phys. Chem. B 1998, 102, 5955–5956. [Google Scholar] [CrossRef]
- Venkataraman, N.V.; Bhagyalakshmi, S.; Vasudevan, S.; Seshadri, R. Conformation and orientation of alkyl chains in the layered organic–inorganic hybrids: (CnH2n+1NH3)2PbI4 (n = 12,16,18). Phys. Chem. Chem. Phys. 2002, 4, 4533–4538. [Google Scholar] [CrossRef]
- Corsetti, S.; Rabl, T.; McGloin, D.; Kiefer, J. Intermediate phases during solid to liquid transitions in long-chain n-alkanes. Phys. Chem. Chem. Phys. 2017, 19, 13941–13950. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Praveena, M.; Guha, K.; Ravishankar, A.; Biswas, S.K.; Bain, C.D.; Jayaram, V. Total internal reflection Raman spectroscopy of poly(alpha-olefin) oils in a lubricated contact. RSC Adv. 2014, 4, 22205–22213. [Google Scholar] [CrossRef]
- Olf, H.G.; Fanconi, B. Low frequency Raman-active lattice vibration of n-paraffins. J. Chem. Phys. 1973, 59, 534–544. [Google Scholar] [CrossRef]
- Kobayashi, M.; Koizumi, H.; Cho, Y. Molecular motion of n-alkane chains in urea inclusion adducts studied by analysis of Raman band profiles. J. Chem. Phys. 1990, 93, 4659–4672. [Google Scholar] [CrossRef]












| Sample | D-LAM | LAM | C–C Mode | |
|---|---|---|---|---|
| Wavenumber, cm−1 | Wavenumber, cm−1 | Wavenumber, cm−1 | FWHM 1, cm−1 | |
| 1-hexene oligomers | ||||
| H2H, C12H26, n = 12, k = 2 2 | 243 | Lines are not observed at room temperature | 1142, 1154 (sh) 3 | 16, 21 |
| H3H, C18H38, n = 18, k = 3 | 218 | 1140 (sh)/1154 | 27 | |
| H4H, C24H50, n = 24, k = 4 | 198 | 1148 | 30 | |
| H5H, C30H62, n = 30, k = 5 | 192 | 1148 | 30 | |
| 1-octene oligomers | ||||
| O2H, C16H34, n = 16, k = 2 | 224 | Lines are not observed at room temperature | 1138, 1152 | 12, 21 |
| O3H, C24H50, n = 24, k = 3 | 187 | 1142 | 33 | |
| O4H, C32H66, n = 32, k = 4 | 175 | 1141 | 33 | |
| O5H, C40H82, n = 40, k = 5 | 173 | 1142 | 33 | |
| Linear alkanes (k = 1) | ||||
| C6H14, n = 6 | 317/330 | 370 | 1142 | 10 |
| C8H18, n = 8 | 296 | 280 | 1137 | 13 |
| C11H24, n = 11 | 243 | 221 | 1134 | 11 |
| C12H26, n = 12 | 237 | 212 | 1134 | 11 |
| C15H32, n = 15 | 223 | Lines are not observed at room temperature | 1133 | 11 |
| C18H38, n = 18 | Absent at room temperature | 133 4 | 1130 | 7 |
| Characteristics | Conformations | |||
|---|---|---|---|---|
| Extended | Branched 1 | Branched 2 | ||
| ΔE, kcal/mol | 0 | 0.112 | 0.112 | |
| ΔE0, kcal/mol | 0 | 0.144 | 0.145 | |
| C–C bond length, Å | 1.54 | 1.54 | 1.54 | |
| Torsion angle (terminal), ° | C1–C2–C3–C4 | −180.0 | −179.3 | 179.7 |
| Torsion angles (central), ° trans−gauche−gauche−trans part | C2–C3–C4–C5 | −175.3 | 178.7 | −177.3 |
| C3–C4–C5–C7 | 168.3 | 72.8 | 165.3 | |
| C3–C4–C5–C6 | −65.8 | −161.5 | −70.0 | |
| C6–C5–C7–C8 | 65.9 | 70.5 | 161.6 | |
| C4–C5–C7–C8 | −168.2 | −164.8 | −72.7 | |
| C5–C7–C8–C9 | 175.6 | 177.8 | −178.7 | |
| Torsion angle (terminal), ° | C7–C8–C9–C10, C8–C9–C10–C11, C9–C10–C11–C12 | 59.9 | −59.9 | 179.8 |
| νC-C, cm−1 (RSA, Å4/amu) | 1139 (17.265) | 1143 (5.87) | 1142 (8.64) | |
| 1149 (8.87) | 1150 (6.34) | |||
| Characteristics | Conformations | |||
|---|---|---|---|---|
| Extended | Branched 1 | Branched 2 | ||
| ΔE, kcal/mol | 0 | 0.108 | 0.107 | |
| ΔE0, kcal/mol | 0 | 0.145 | 0.157 | |
| C–C bond length, Å | 1.53 | 1.54 | 1.54 | |
| Torsion angle (terminal), ° | C1–C2–C3–C4, C2–C3–C4–C5, C3–C4–C5–C6 | −180.0 | −179.7 | 59.9 |
| Torsion angle (central), ° trans-gauche-gauche-trans part | C4–C5–C6–C7 | −175.5 | 178.9 | −177.8 |
| C5–C6–C7–C9 | 168.0 | 72.9 | 164.8 | |
| C5–C6–C7–C8 | −66.0 | −161.4 | −70.5 | |
| C8–C7–C9–C10 | 66.0 | 70.5 | 161.5 | |
| C6–C7–C9–C10 | −168.0 | −164.8 | −72.8 | |
| C7–C9–C10–C11 | 175.5 | 177.8 | −178.9 | |
| Torsion angle (terminal), ° | C9–C10–C11–C12, C10–C11–C12–C13, C11–C12–C13–C14, C12–C13–C14–C15, C13–C14–C15–C16 | 180.0 | 36.1 | 179.8 |
| νC–C, cm−1 (RSA, Å4/amu) | 1136 (27.87) | 1140 (8.16) | 1140 (11.62) | |
| 1146 (13.24) | 1147 (10.80) | |||
| Samples | νas(CH2) cm−1 | νas(CH3) cm−1 | Samples | νas(CH2), cm−1 | νas(CH3), cm−1 |
|---|---|---|---|---|---|
| 1-hexene oligomers | 1-octene oligomers | ||||
| H2H, C12H26, n = 12, k = 2 | 2892 | 2960 | O2H, C16H34, n = 16, k = 2 | 2890 | 2960 |
| H3H, C18H38, n = 18, k = 3 | 2895 | 2961 | O3H, C24H50, n = 24, k = 3 | 2890 | 2961 |
| H4H, C24H50, n = 24, k = 4 | 2894 | 2960 | O4H, C32H66, n = 32, k = 4 | 2890 | 2960 |
| H5H, C30H62, n = 30, k = 5 | 2895 | 2960 | O5H, C40H82, n = 40, k = 5 | 2891 | 2960 |
| Linear alkanes (k = 1) | |||||
| C6H14, n = 6 | 2895 | 2960 | C8H18, n = 8 | 2894 | 2961 |
| C12H26, n = 12 | 2891 | 2960 | C16H34, n = 16 1 | 2890 | 2961 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuznetsov, S.M.; Iablochnikova, M.S.; Sagitova, E.A.; Prokhorov, K.A.; Nikolaeva, G.Y.; Ustynyuk, L.Y.; Ivchenko, P.V.; Vinogradov, A.A.; Vinogradov, A.A.; Nifant’ev, I.E. Raman Spectroscopy Study of Structurally Uniform Hydrogenated Oligomers of α-Olefins. Polymers 2020, 12, 2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092153
Kuznetsov SM, Iablochnikova MS, Sagitova EA, Prokhorov KA, Nikolaeva GY, Ustynyuk LY, Ivchenko PV, Vinogradov AA, Vinogradov AA, Nifant’ev IE. Raman Spectroscopy Study of Structurally Uniform Hydrogenated Oligomers of α-Olefins. Polymers. 2020; 12(9):2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092153
Chicago/Turabian StyleKuznetsov, Sergey M., Maria S. Iablochnikova, Elena A. Sagitova, Kirill A. Prokhorov, Gulnara Yu. Nikolaeva, Leila Yu. Ustynyuk, Pavel V. Ivchenko, Alexey A. Vinogradov, Alexander A. Vinogradov, and Ilya E. Nifant’ev. 2020. "Raman Spectroscopy Study of Structurally Uniform Hydrogenated Oligomers of α-Olefins" Polymers 12, no. 9: 2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092153