Preparation of Chitosan—Graphene Oxide Composite Aerogel by Hydrothermal Method and Its Adsorption Property of Methyl Orange
Abstract
:1. Introduction
2. Experimental
2.1. Raw Materials
2.2. Preparation of Chitosan (CS) Aerogel by Sol-Gel Method
2.3. Preparation of Graphene Oxide (GO) Aerogel by Hydrothermal Method
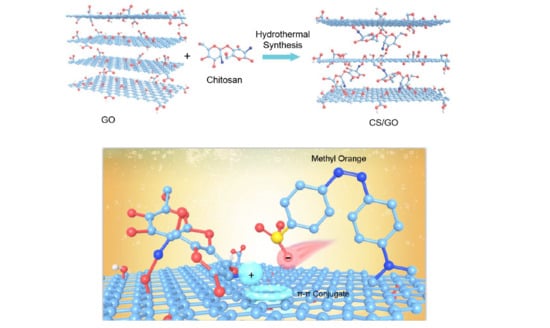
2.4. Preparation of Chitosan/Graphene Oxide (CS/GO) Composite Aerogel by Hydrothermal Method
2.5. Preparation of CS/GO Aerogel by Chemical Crosslinking
2.6. Preparation of CS/GO Aerogel by Sol-Gel Method
2.7. Material Characterizations
2.8. Adsorption Characterization
3. Result and Discussion
3.1. The Structure and Adsorption Properties of CS/GO Aerogels Prepared by Three Experimental Methods
3.2. FTIR Analyses of Various Aerogels
3.3. XRD Analysis of Various Aerogels
3.4. SEM Analyses of Various Aerogels
3.5. Physical Adsorption and Desorption Test of N2
3.6. Effect of Adsorbent Types
3.7. Effect of Raw Material Ratio on the Adsorption of CS/GO Aerogel
3.8. Effect of pH
3.9. Influence of Adsorption Time
3.10. Desorption
4. Adsorbing Mechanism Analysis of CS/GO Aerogel
4.1. Adsorption Kinetics Study
4.2. Isothermal Adsorption Model
4.3. Adsorption Mechanism
5. Conclusions
- (1)
- The hydrothermal method is an acceptable and applicable method to prepare the CS/GO aerogel with a high adsorption rate (96.6%), which proved to be better than the other approaches, such as the sol-gel method (59.45%) and chemical crosslinking method (89.2%);
- (2)
- The microstructural characterizations indicate that the CS/GO aerogel has a combined structure provided by CS and GO aerogels, which has a larger specific surface area (297.431 m2/g), more micro-holes, and higher absorption volume;
- (3)
- The experimental data of the CS/GO aerogel were well fitted by the Langmuir isotherm model in the case of MO. The quasi-secondary adsorption kinetic model provided a better correlation of the adsorption data than the quasi-first adsorption kinetic model, implying that the adsorption process is controlled by the active site of chemisorption;
- (4)
- The CS/GO aerogel can enhance the adsorption capacity of MO and it can be well used for the adsorption of MO in a larger pH range (pH = 1~9); and
- (5)
- CSGO is a promising adsorbent for the adsorption of MO in practice. Despite all this, this study mainly focused on the adsorption properties of CSGO for MO, and not for other sources of contaminants. Therefore, future research should be more concerned with cationic dyes and heavy metals.
Author Contributions
Funding
Conflicts of Interest
References
- Srivastava, V.; Maydannik, P.; Sharma, Y.C.; Sillanpaa, M. Synthesis and application of polypyrrole coated tenorite nanoparticles (PPy@TN) for the removal of anionic food dye ‘Tartrazine’ and divalent metallic ions viz. Pb(II), Cd(II), Zn(II), Co(II), Mn(II) from synthetic wastewater. RSC Adv. 2015, 5, 80829–80843. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.L.; Li, C.J.; Liu, S.; Zhang, F.Q.; You, F.; Yao, C. The synthesis and characterization of ytterbium-doped TiO2 hollow spheres with enhanced visible-light photocatalytic activity. RSC Adv. 2017, 7, 24598–24606. [Google Scholar] [CrossRef] [Green Version]
- Zhan, C.B.; Li, Y.X.; Sharma, P.R.; He, H.R.; Sharma, S.K.; Wang, R.F.; Hsiao, B.S. A study of TiO2 nanocrystal growth and environmental remediation capability of TiO2/CNC nanocomposites. RSC Adv. 2019, 9, 40565–40576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sharma, P.R.; Chattopadhyay, A.; Sharma, S.K.; Geng, L.H.; Amiralian, N.; Martin, D.; Hsiao, B.S. Nanocellulose from Spinifex as an Effective Adsorbent to Remove Cadmium(II) from Water. ACS Sustain. Chem. Eng. 2018, 6, 3279–3290. [Google Scholar] [CrossRef]
- Long, Q.; Zhang, Z.; Qi, G.X.; Wang, Z.; Chen, Y.B.; Liu, Z.Q. Fabrication of Chitosan Nanofiltration Membrane by Film Casting Strategy for Effective Removal of Dye/Salt in Textile Wastewater. ACS Sustain. Chem. Eng. 2020, 8, 2512–2522. [Google Scholar] [CrossRef]
- Ding, Y.L.; Tian, Z.; Li, H.J.; Wang, X.M. Efficient removal of organic dyes using a three-dimensional graphene aerogel with excellent recycling stability. New Carbon Mater. 2019, 34, 315–324. [Google Scholar] [CrossRef]
- Ma, Y.; Yu, F.; Ma, J. Design of Graphene/Organic Composite Adsorbent and Its Application in Water Treatment. Prog. Chem. 2017, 29, 582–592. [Google Scholar]
- Liao, Y.; Wang, M.; Chen, D.J. Preparation of polydopamine-modified graphene oxide/chitosan aerogel for uranium(VI) adsorption. Ind. Eng. Chem. Res. 2018, 57, 8472–8483. [Google Scholar] [CrossRef]
- Ghasemi, O.; Mehrdadi, N.; Baghdadi, M.; Aminzadeh, B.; Ghaseminejad, A. Spilled oil absorption from Caspian sea water by graphene/chitosan nano composite. Energy Sources Part A Recovery Util. Environ. Eff. 2019, 55, 1–17. [Google Scholar] [CrossRef]
- Liu, L.; Li, C.; Bao, C.L.; Jia, Q.; Xiao, P.F.; Liu, X.T.; Zhang, Q.P. Preparation and characterization of chitosan/graphene oxide composites for the adsorption of Au(III) and Pd(II). Talanta 2012, 93, 350–357. [Google Scholar] [CrossRef]
- Sabzevari, M.; Cree, D.E.; Wilson, L.D. Graphene Oxide–Chitosan Composite Material for Treatment of a Model Dye Effluent. ACS Omega 2018, 3, 13045–13054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Xia, G.M.; Wu, C.; Sun, J.; Song, R.; Huang, W. Porous chitosan aerogels doped with graphene oxide as highly effective adsorbent for methyl orange and amido black 10B. Carbohydr. Polym. 2015, 115, 686–693. [Google Scholar] [CrossRef]
- Jiang, X.L.; Yu, L.; Yao, C.; You, F.; Zhang, J. Facile Fabrication and Characterization of Ytterbium Oxide Hollow Spheres using Carbon Spheres as Template. Nano 2016, 11, 1650067. [Google Scholar] [CrossRef]
- Samuels, R.J. Solid state characterization of the structure of chitosan films. J. Polym. Sci. Part A Polym. Chem. 1981, 19, 1081–1105. [Google Scholar] [CrossRef]
- Liu, F.F.; Fan, J.L.; Wang, S.G.; Ma, G.H. Adsorption of natural organic matter analogues by multi-walled carbon nanotubes: Comparison with powdered activated carbon. Chem. Eng. J. 2013, 219, 450–458. [Google Scholar] [CrossRef]
- Saha, T.K.; Bhoumik, N.C.; Karmaker, S.; Ahmed, M.G.; Ichikawa, H.; Fukumori, Y. Adsorption of Methyl Orange onto Chitosan from Aqueous Solution. J. Water Resour. Prot. 2010, 2, 898–906. [Google Scholar] [CrossRef] [Green Version]
- Li, H.M.; Deng, B.J.; Li, C.X. Several Regeneration Methods of Activated Carbon. Technol. Dev. Chem. Ind. 2006, 11, 21–24. [Google Scholar]
- Bi, H.; Xie, X.; Yin, K.; Zhou, Y.; Wan, S.; He, L.; Xu, F.; Banhart, F.; Sun, L.; Ruoff, R.S. Spongy Graphene as a Highly Efficient and Recyclable Sorbent for Oils and Organic Solvents. Adv. Funct. Mater. 2012, 21, 4421–4425. [Google Scholar] [CrossRef]
- Sharif, F.; Gagnon, L.R.; Mulmi, S.; Roberts, E.P.L. Electrochemical regeneration of a reduced graphene oxide/magnetite composite adsorbent loaded with methylene blue. Water Res. A J. Int. Water Assoc. 2017, 114, 237–245. [Google Scholar] [CrossRef]
- Silva, J.M.; Ribeiro, L.S.; Órfão, J.J.M.; Soria, M.A.; Madeira, L.M. Low temperature glycerol steam reforming over a Rh-based catalyst combined with oxidative regeneration. Int. J. Hydrog. Energy 2019, 44, 2461–2473. [Google Scholar] [CrossRef]
- Ho, Y.S.; McKay, G. Pseudo-second order model for sorption processes. Process Biochem. 1999, 34, 451–465. [Google Scholar] [CrossRef]
- Zhao, L.Q.; Yang, S.N.; Yilihamu, A.; Ma, Q.; Shi, M.Y.; Ouyang, B.W.; Zhang, Q.Q.; Guan, X.; Yang, S.T. Adsorptive decontamination of Cu2+-contaminated water and soil by carboxylated graphene oxide/chitosan/cellulose composite beads. Environ. Res. 2019, 179 Pt A, 108779. [Google Scholar] [CrossRef]
- Langmuir, I. The adsorption of gases on plane surfaces of glass, mica and platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef] [Green Version]
- Freunidlich, H.M.F. Over the adsorption in solution. J. Phys. Chem. 1906, 57, 385–471. [Google Scholar]
- Zamani, S.; Tabrizi, N.S. Removal of methylene blue from water by graphene oxide aerogel: Thermodynamic, kinetic, and equilibrium modeling. Res. Chem. Intermed. 2014, 41, 7945–7963. [Google Scholar] [CrossRef]
- Yan, Y.H.; Li, H.J.; Yang, H.; Li, A.M.; Cheng, R.S. Removal of various cationic dyes from aqueous solutions using a kind of fully biodegradable magnetic composite microsphere. Chem. Eng. J. 2013, 223, 402–411. [Google Scholar] [CrossRef]
- Zhang, Z.X.; Liu, X.; Liu, Y.; Deng, L.H. Construction of drug carrier for go modified chitosan. Polym. Mater. Sci. Eng. 2019, 35, 136–143. [Google Scholar]
- Singh, N.; Riyajuddin, S.; Ghosh, K.; Mehta, S.K.; Dan, A. Chitosan-Graphene Oxide Hydrogels with Embedded Magnetic Iron Oxide Nanoparticles for Dye Removal. ACS Appl. Nano Mater. 2019, 2, 7379–7392. [Google Scholar] [CrossRef]
- Yu, R.M.; Shi, Y.Z.; Yang, D.Z.; Liu, Y.X.; Qu, J.; Yu, Z.Z. Graphene Oxide/Chitosan Aerogel Microspheres with Honeycomb-Cobweb and Radially Oriented Microchannel Structures for Broad-Spectrum and Rapid Adsorption of Water Contaminants. ACS Appl. Mater. Interfaces 2017, 9, 21809–21819. [Google Scholar] [CrossRef]
- Liu, F.; Chung, S.Y.; Oh, G.; Seo, T.S. Three-Dimensional Graphene Oxide Nanostructure for Fast and Efficient Water-Soluble Dye Removal. ACS Appl. Mater. Interfaces 2012, 4, 922–927. [Google Scholar] [CrossRef]
- Yang, Q.X.; Lu, R.; Ren, S.S.; Chen, C.T.; Chen, Z.J.; Yang, X.Y. Three dimensional reduced graphene oxide/ZIF-67 aerogel: Effective removal cationic and anionic dyes from water. Chem. Eng. J. 2018, 348, 202–211. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhu, X.Y.; Chen, B.L. Stable graphene oxide/poly(ethyleneimine) 3D aerogel with tunable surface charge for high performance selective removal of ionic dyes from water. Chem. Eng. J. 2018, 334, 1119–1127. [Google Scholar] [CrossRef]
- Zhang, Y.Y.; Yang, X.R.; Yan, Y.Y.; Chen, D.J.; Huang, L.H.; Zhang, J.X.; Ke, Y.; Tan, S.Z. The utilization of a three-dimensional reduced graphene oxide and montmorillonite composite aerogel as a multifunctional agent for wastewater treatment. RSC Adv. 2018, 8, 4239–4248. [Google Scholar] [CrossRef] [Green Version]
- Zhu, H.Y.; Jiang, R.; Xiao, L.; Zeng, G.M. Preparation, characterization, adsorption kinetics and thermodynamics of novel magnetic chitosan enwrapping nanosized γ-Fe2O3 and multi-walled carbon nanotubes with enhanced adsorption properties for methyl orange. Bioresour. Technol. 2010, 101, 5063–5069. [Google Scholar] [CrossRef] [PubMed]
- Yan, H. Adsorption and Removal of Various Pollutants in Water by Graphene Materials. Ph.D. Thesis, Nanjing University, Nanjing, China, 2015. [Google Scholar]
- Zhang, X.P.; Liu, D.; Yang, L.; Zhou, L.M.; You, T.Y. Self-assembled three-dimensional graphene-based materials for dye adsorption and catalysis. J. Mater. Chem. A 2015, 3, 10031–10037. [Google Scholar] [CrossRef]
- Chen, C.; Zhu, X.Y.; Chen, B.L. Covalently cross-linked graphene oxide aerogel with stable structure for high-efficiency water purification. Chem. Eng. J. 2018, 354, 896–904. [Google Scholar] [CrossRef]
- Jiang, R.; Fu, Y.Q.; Zhu, H.Y.; Yao, J.; Xiao, L. Removal of methyl orange from aqueous solutions by magnetic maghemite/chitosan nanocomposite films: Adsorption kinetics and equilibrium. J. Appl. Polym. Sci. 2012, 125, 540–549. [Google Scholar] [CrossRef]












| Sample | BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| CS/GOS | 56.916 | 0.267 | 15.275 |
| CS/GOc | 147.49 | 0.680 | 13.609 |
| CS/GO | 297.431 | 0.238 | 3.057 |
| Sample | BET (m2/g) | Pore Volume (cm3/g) | Pore Diameter (nm) |
|---|---|---|---|
| CS | 88.993 | 0.1254 | 5.64 |
| GO | 31.88 | 0.137 | 8.287 |
| CS/GO | 297.431 | 0.238 | 3.057 |
| Model | k/(mg·g−1·min−1) | qe/(mg·g−1) | R2 |
|---|---|---|---|
| quasi-first-order adsorption kinetics model | 0.01219 | 30.79 | 0.96239 |
| quasi-secondary adsorption kinetics model | 0.000641 | 52.6 | 0.99527 |
| Model | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| t/°C | qmax (mg·g−1) | kL (L/mg) | R2 | kF (mg/g (L/mg)1/n) | n | R2 |
| 25 | 38.67 | 5.002 | 0.9932 | 28.84 | 10.55 | 0.81199 |
| Concentration (mg/L) | 10 | 20 | 40 | 50 |
|---|---|---|---|---|
| RL | 0.0196 | 0.0099 | 0.00498 | 0.00398 |
| Adsorbents | qe (mg/g) | BET (m2/g) | Pore Diameter (nm) | Experimental Methods | References |
|---|---|---|---|---|---|
| GP55 | 30 | 453.4 | 3–4 | Chemical crosslinking | [32] |
| RGO-MMT | 32.5 | 43.17 | 123.28 | Sol-gel method | [33] |
| M-CS/γ-Fe2O3/MWCNTs | 31.44 | — | — | Chemical crosslinking | [34] |
| CSGO | 32.73 | — | — | Sol-gel method | [35] |
| L-Cys-GA | 75 | 154 | 3.7 | Template method | [36] |
| GCAs | 20 | 68.11 | — | Chemical crosslinking | [37] |
| γ-Fe2O3/chitosan composite films | 29.41 | — | — | solution casting method | [38] |
| CS/GO | 48.6 | 297.431 | 3 | Hydrothermal method | this study |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, W.; Jiang, X.; Liu, F.; You, F.; Yao, C. Preparation of Chitosan—Graphene Oxide Composite Aerogel by Hydrothermal Method and Its Adsorption Property of Methyl Orange. Polymers 2020, 12, 2169. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092169
Zhu W, Jiang X, Liu F, You F, Yao C. Preparation of Chitosan—Graphene Oxide Composite Aerogel by Hydrothermal Method and Its Adsorption Property of Methyl Orange. Polymers. 2020; 12(9):2169. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092169
Chicago/Turabian StyleZhu, Wei, Xueliang Jiang, Fangjun Liu, Feng You, and Chu Yao. 2020. "Preparation of Chitosan—Graphene Oxide Composite Aerogel by Hydrothermal Method and Its Adsorption Property of Methyl Orange" Polymers 12, no. 9: 2169. https://0-doi-org.brum.beds.ac.uk/10.3390/polym12092169