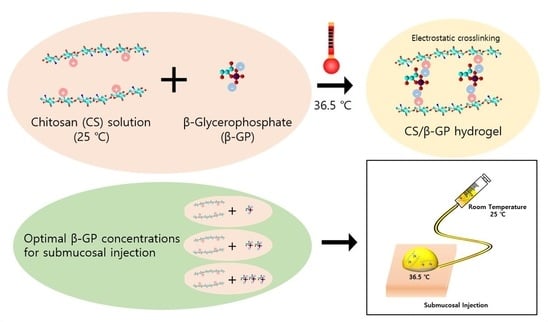
Injectable Thermosensitive Chitosan Solution with β-Glycerophosphate as an Optimal Submucosal Fluid Cushion for Endoscopic Submucosal Dissection
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of the Thermosensitive Chitosan Solution
2.2. Thermo-Sensitivity Evaluation
2.3. Rheological Evaluation
2.4. Injectability Evaluation
2.5. Cytotoxicity
2.6. Preclinical Evaluation
2.6.1. Animal Model
2.6.2. Experiment Procedure
2.7. Scanning Electron Microscope (SEM) Specimen
2.8. Statistical Analysis
3. Results
3.1. Thermo-Sensitivity of Solutions
3.2. Rheological Evaluation
3.2.1. Temperature Sweep
3.2.2. Time Sweep
3.3. Injectability Evaluation
3.4. Biocompatibility Evaluation
3.5. Preclinical Evaluation
3.6. SEM Surface Examination
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chenite, A.; Chaput, C.; Wang, D.; Combes, C.; Buschmann, M.D.; Hoemann, C.D.; Leroux, J.C.; Atkinson, B.L.; Binette, F.; Selmani, A. Novel injectable neutral solutions of chitosan form biodegradable gels in situ. Biomaterials 2000, 21, 2155–2161. [Google Scholar] [CrossRef]
- Park, S.; Chun, H.J.; Kim, C.Y.; Kim, J.Y.; Jang, J.S.; Kwon, Y.D.; Kim, D.R.; Keum, B.; Seo, Y.S.; Kim, Y.S.; et al. Electrical characteristics of various submucosal injection fluids for endoscopic mucosal resection. Dig. Dis. Sci. 2008, 53, 1678–1682. [Google Scholar] [CrossRef]
- Cho, K.B.; Jeon, W.J.; Kim, J.J. Worldwide experiences of endoscopic submucosal dissection: Not just Eastern acrobatics. World J. Gastroenterol. 2011, 17, 2611–2617. [Google Scholar] [CrossRef] [PubMed]
- Kim, E.R.; Park, Y.G.; Min, B.H.; Lee, J.H.; Rhee, P.L.; Kim, J.J.; Park, J.H.; Park, D.I.; Chang, D.K. Usefulness of ready-to-Use 0.4% sodium hyaluronate (Endo-Ease) in the endoscopic resection of gastrointestinal neoplasms. Clin. Endosc. 2015, 48, 392–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, L.; Li, Q.; Zhang, C.; Wu, H.; Yao, L.; Xu, M.; Yu, L.; Ding, J. Safe and Efficient Colonic Endoscopic Submucosal Dissection Using an Injectable Hydrogel. ACS Biomater. Sci. Eng. 2016, 2, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Feitoza, A.B.; Gostout, C.J.; Burgart, L.J.; Burkert, A.; Herman, L.J.; Rajan, E. Hydroxypropyl methylcellulose: A better submucosal fluid cushion for endoscopic mucosal resection. Gastrointest. Endosc. 2003, 57, 41–47. [Google Scholar] [CrossRef]
- Yamamoto, H.; Yube, T.; Isoda, N.; Sato, Y.; Sekine, Y.; Higashizawa, T.; Ido, K.; Kimura, K.; Kanai, N. A novel method of endoscopic mucosal resection using sodium hyaluronate. Gastrointest. Endosc. 1999, 50, 251–256. [Google Scholar] [CrossRef]
- Castro, R.; Libânio, D.; Pita, I.; Dinis-Ribeiro, M. Solutions for submucosal injection: What to choose and how to do it. World J. Gastroenterol. 2019, 25, 777–788. [Google Scholar] [CrossRef]
- Nagelschmidt, M. Endoscopic use of fibrin adhesives: Problems when injecting through long catheters. Surg. Endosc. 1999, 13, 80–82. [Google Scholar] [CrossRef]
- Cypher, L.; Sun, S.; Forster, E.; Hoffman, B.; Lewin, D. Submucosal Lifting Agent ORISE Gel Remnants Histopathologically Mimic Mucin and Malignancy: A Case Series. Am. J. Clin. Pathol. 2019, 152, S73. [Google Scholar] [CrossRef]
- Lammel-Lindemann, J.; Dourado, I.A.; Shanklin, J.; Rodriguez, C.A.; Catalani, L.H.; Dean, D. Photocrosslinking-based 3D printing of unsaturated polyesters from isosorbide: A new material for resorbable medical devices. Bioprinting 2019, 18, e00062. [Google Scholar] [CrossRef]
- Yu, L.; Xu, W.; Shen, W.; Cao, L.; Liu, Y.; Li, Z.; Ding, J. Poly(lactic acid-co-glycolic acid)-poly(ethylene glycol)-poly(lactic acid-co-glycolic acid) thermogel as a novel submucosal cushion for endoscopic submucosal dissection. Acta Biomater. 2014, 10, 1251–1258. [Google Scholar] [CrossRef] [PubMed]
- Nagase, K.; Yamato, M.; Kanazawa, H.; Okano, T. Poly(N-isopropylacrylamide)-based thermoresponsive surfaces provide new types of biomedical applications. Biomaterials 2018, 153, 27–48. [Google Scholar] [CrossRef] [PubMed]
- Doberenz, F.; Zeng, K.; Willems, C.; Zhang, K.; Groth, T. Thermoresponsive polymers and their biomedical application in tissue engineering—A review. J. Mater. Chem. B 2020, 8, 607–628. [Google Scholar] [CrossRef] [PubMed]
- Haq, M.A.; Su, Y.; Wang, D. Mechanical properties of PNIPAM based hydrogels: A review. Mater. Sci. Eng. C 2017, 70, 842–855. [Google Scholar] [CrossRef]
- Akash, M.S.H.; Rehman, K. Recent progress in biomedical applications of Pluronic (PF127): Pharmaceutical perspectives. J. Control. Release 2015, 209, 120–138. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.S. Photo-cross-linkable and thermo-responsive hydrogels containing chitosan and Pluronic for sustained release of human growth hormone (hGH). J. Biomater. Sci. Polym. Ed. 2007, 18, 1429–1441. [Google Scholar] [CrossRef] [PubMed]
- Rohani Rad, E.; Vahabi, H.; Formela, K.; Saeb, M.R.; Thomas, S. Injectable poloxamer/graphene oxide hydrogels with well-controlled mechanical and rheological properties. Polym. Adv. Technol. 2019, 30, 2250–2260. [Google Scholar] [CrossRef]
- Chandy, T.; Sharma, C.P. Chitosan-as a Biomaterial. Biomater. Artif. Cells Artif. Organs 1990, 18, 1–24. [Google Scholar] [CrossRef]
- Ta, H.T.; Han, H.; Larson, I.; Dass, C.R.; Dunstan, D.E. Chitosan-dibasic orthophosphate hydrogel: A potential drug delivery system. Int. J. Pharm. 2009, 371, 134–141. [Google Scholar] [CrossRef]
- Liu, L.; Tang, X.; Wang, Y.; Guo, S. Smart gelation of chitosan solution in the presence of NaHCO 3 for injectable drug delivery system. Int. J. Pharm. 2011, 414, 6–15. [Google Scholar] [CrossRef] [PubMed]
- Aliaghaei, M.; Mirzadeh, H.; Dashtimoghadam, E.; Taranejoo, S. Investigation of gelation mechanism of an injectable hydrogel based on chitosan by rheological measurements for a drug delivery application. Soft Matter 2012, 8, 7128–7137. [Google Scholar] [CrossRef]
- Talaat, W.M.; Haider, M.; Al Kawas, S.; Kandil, N.G.; Harding, D.R.K. Chitosan-based thermosensitive hydrogel for controlled drug delivery to the temporomandibular joint. J. Craniofac. Surg. 2016, 27, 735–740. [Google Scholar] [CrossRef] [PubMed]
- Park, S.; Chun, H.J.; Kwon, Y.D.; Keum, B.; Seo, Y.S.; Kim, Y.S.; Jeen, Y.-T.; Um, S.H.; Kim, C.D.; Ryu, H.S.; et al. Stretching Causes Extensive Changes of Gastric Submucosa: Is It Acceptable to Define 500μm as the Safe Margin? Gut Liver 2008, 2, 199–204. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Nishimoto, S.K.; Bumgardner, J.D.; Haggard, W.O.; Gaber, M.W.; Yang, Y. A chitosan/β-glycerophosphate thermo-sensitive gel for the delivery of ellagic acid for the treatment of brain cancer. Biomaterials 2010, 31, 4157–4166. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Dong, A.; Yang, J.; Deng, L. Preparation and properties of an injectable thermo-sensitive double crosslinking hydrogel based on thiolated chitosan/beta-glycerophosphate. J. Mater. Sci. 2012, 47, 2509–2517. [Google Scholar] [CrossRef]
- Niranjan, R.; Koushik, C.; Saravanan, S.; Moorthi, A.; Vairamani, M.; Selvamurugan, N. A novel injectable temperature-sensitive zinc doped chitosan/β-glycerophosphate hydrogel for bone tissue engineering. Int. J. Biol. Macromol. 2013, 54, 24–29. [Google Scholar] [CrossRef]
- Zhou, H.Y.; Jiang, L.J.; Cao, P.P.; Li, J.B.; Chen, X.G. Glycerophosphate-based chitosan thermosensitive hydrogels and their biomedical applications. Carbohydr. Polym. 2015, 117, 524–536. [Google Scholar] [CrossRef]
- Jeon, H.J.; Jeong, S.; Choi, H.S.; Jang, S.H.; Kim, S.H.; Kim, S.H.; Lee, J.M.; Sun, E.; Keum, B.; Jeen, Y.T.; et al. A Comparative Study on Aqueous Chitosan Solution and Various Submucosal Injection Fluids Using a Three-Dimensional Sensor. Gut Liver 2021, 15, 217. [Google Scholar] [CrossRef]
- Chung, I.-K.; Lee, J.H.; Lee, S.-H.; Kim, S.-J.; Cho, J.Y.; Cho, W.Y.; Hwangbo, Y.; Keum, B.R.; Park, J.J.; Chun, H.-J.; et al. Therapeutic outcomes in 1000 cases of endoscopic submucosal dissection for early gastric neoplasms: Korean ESD Study Group multicenter study. Gastrointest. Endosc. 2009, 69, 1228–1235. [Google Scholar] [CrossRef]
- Jeon, S.W.; Jung, M.K.; Cho, C.M.; Tak, W.Y.; Kweon, Y.O.; Kim, S.K.; Choi, Y.H. Predictors of immediate bleeding during endoscopic submucosal dissection in gastric lesions. Surg. Endosc. 2009, 23, 1974–1979. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Zhang, D.Y.; Lu, S.T.; Li, P.W.; Li, S.D. Chitosan-based composite materials for prospective hemostatic applications. Mar. Drugs 2018, 16, 273. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.; Zhou, T.; Liu, J.; Wan, Y. Injectable chitosan/dextran-polylactide/glycerophosphate hydrogels and their biodegradation. Polym. Degrad. Stab. 2015, 120, 273–282. [Google Scholar] [CrossRef]
- Xu, X.; Gu, Z.; Chen, X.; Shi, C.; Liu, C.; Liu, M.; Wang, L.; Sun, M.; Zhang, K.; Liu, Q.; et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin. Acta Biomater. 2019, 86, 235–246. [Google Scholar] [CrossRef] [PubMed]
- Vandamme, T.; Schoch, C.; Riemenschnitter, M.; Anton, N.; Supper, S.; Seidel, N. Rheological Study of Chitosan/Polyol-phosphate Systems: Influence of the Polyol Part on the Thermo-Induced Gelation Mechanism. Langmuir 2013, 29, 10229–10237. [Google Scholar] [CrossRef]
- Jin Hyun, J.; Rae Chun, H.; Jai Chun, H.; Tae Jeen, Y.; Won Baeck, C.; Kyun Yu, S.; Sik Kim, Y.; Sik Lee, H.; Ho Um, S.; Woo Lee, S.; et al. Comparison of the characteristics of submucosal injection solutions used in endoscopic mucosal resection. Scand. J. Gastroenterol. 2006, 41, 488–492. [Google Scholar] [CrossRef] [PubMed]
- Pioche, M.; Ciocirlan, M.; Lépilliez, V.; Salmon, D.; Mais, L.; Guillaud, O.; Hervieu, V.; Petronio, M.; Lienhart, I.; Adriano, J.-L.; et al. High-pressure jet injection of viscous solutions for endoscopic submucosal dissection: A study on ex vivo pig stomachs. Surg. Endosc. 2014, 28, 1742–1747. [Google Scholar] [CrossRef]
- International Organization for Standardization. Biological Evaluation of Medical Devices—Part 5: In Vitro Cytotoxicity Testing; EN ISO 10993; International Organization for Standardization: Geneva, Switzerland, 2009; pp. 1–13. [Google Scholar]
- Jain, A.; Rogojevic, S.; Ponoth, S.; Agarwal, N.; Matthew, I.; Gill, W.N.; Persans, P.; Tomozawa, M.; Plawsky, J.L.; Simonyi, E. Porous silica materials as low-k dielectrics for electronic and optical interconnects. Thin Solid Films 2001, 398–399, 513–522. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, S.; Jeon, H.J.; Jang, K.-J.; Park, S.; Choi, H.S.; Chung, J.H. Injectable Thermosensitive Chitosan Solution with β-Glycerophosphate as an Optimal Submucosal Fluid Cushion for Endoscopic Submucosal Dissection. Polymers 2021, 13, 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111696
Jeong S, Jeon HJ, Jang K-J, Park S, Choi HS, Chung JH. Injectable Thermosensitive Chitosan Solution with β-Glycerophosphate as an Optimal Submucosal Fluid Cushion for Endoscopic Submucosal Dissection. Polymers. 2021; 13(11):1696. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111696
Chicago/Turabian StyleJeong, Seung, Han Jo Jeon, Kyoung-Je Jang, Sangbae Park, Hyuk Soon Choi, and Jong Hoon Chung. 2021. "Injectable Thermosensitive Chitosan Solution with β-Glycerophosphate as an Optimal Submucosal Fluid Cushion for Endoscopic Submucosal Dissection" Polymers 13, no. 11: 1696. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111696