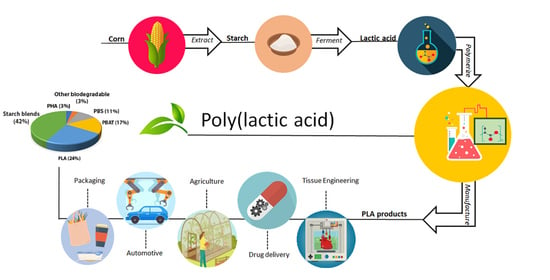
Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications
Abstract
:1. Introduction
2. Synthesis of Monomers
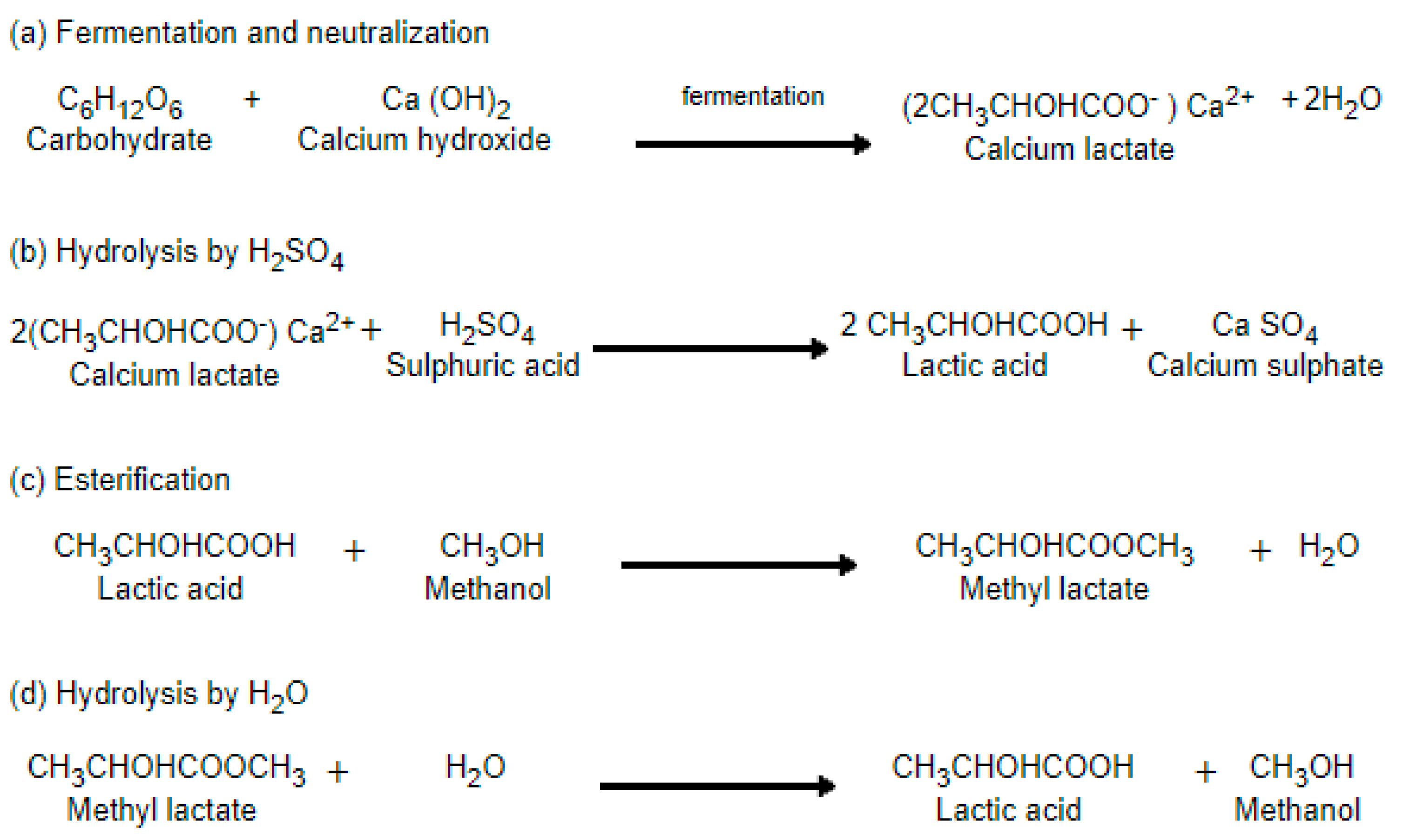
2.1. Synthesis of Lactic Acid
2.2. Synthesis of Lactide Monomer
3. Synthesis of PLA
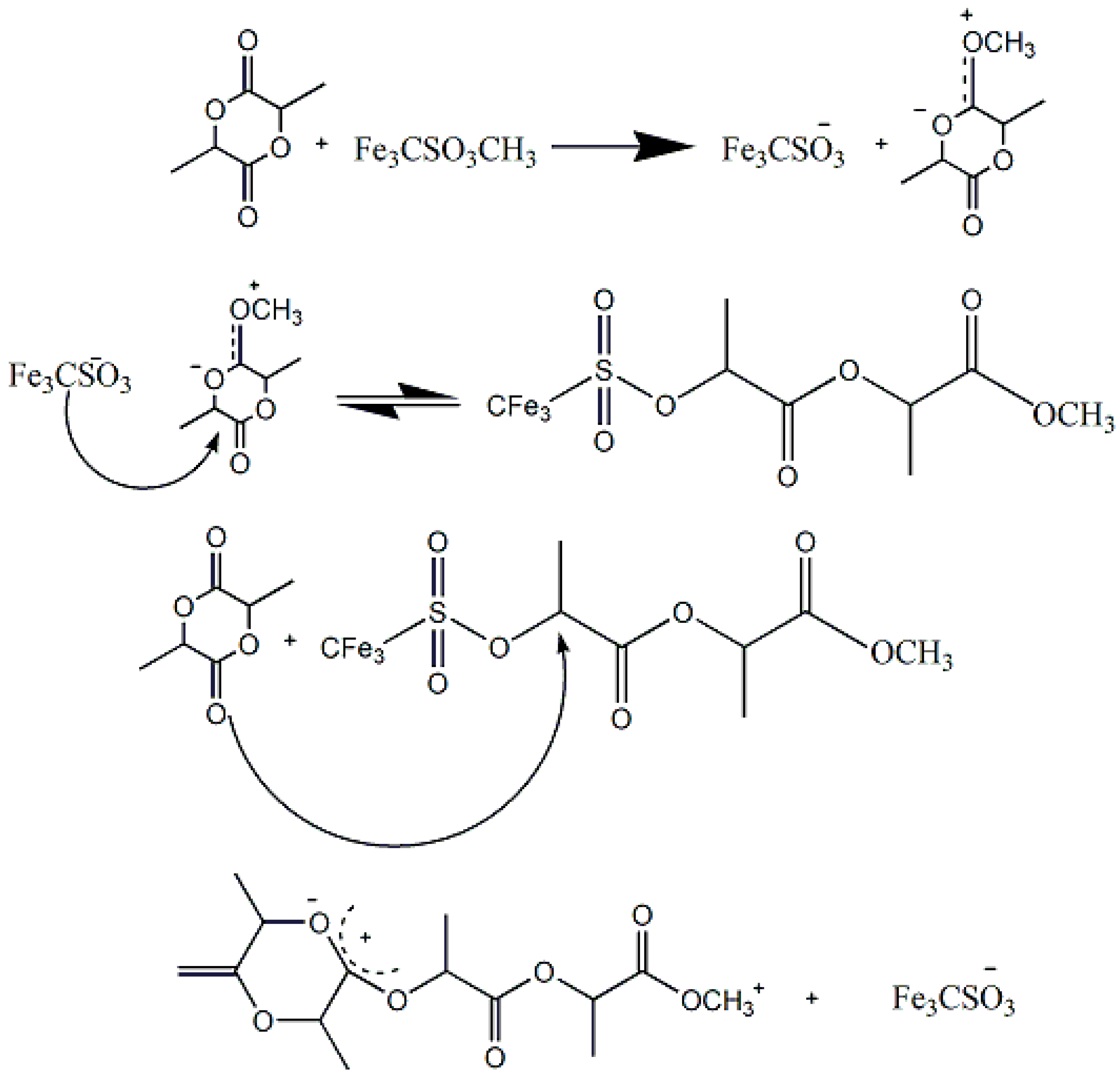
3.1. PLA Synthesis via Polycondensation of Lactic Acid
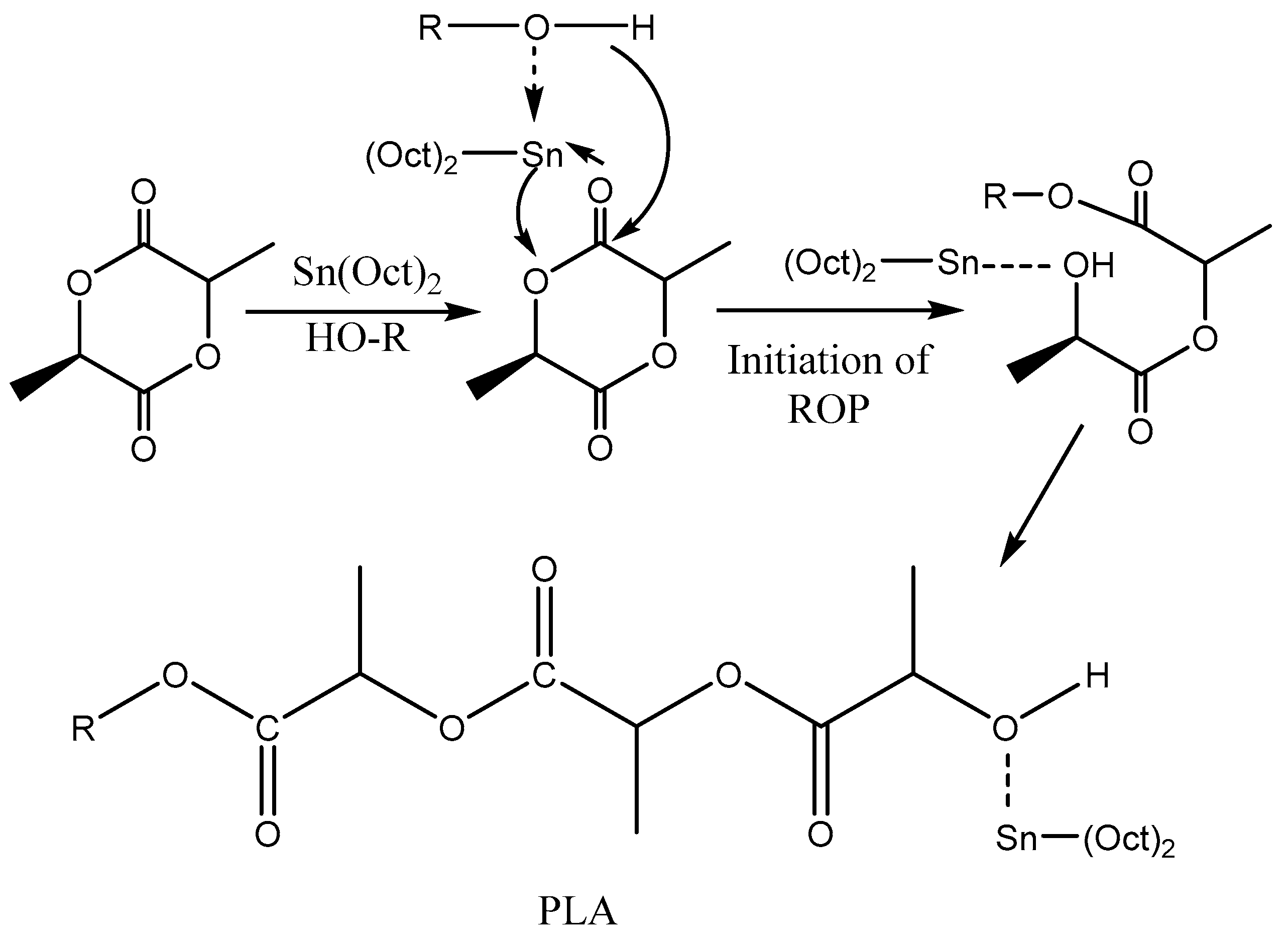
3.2. Ring—Opening Polymerization of Lactide
3.2.1. Mechanisms of Ring-Opening Polymerization
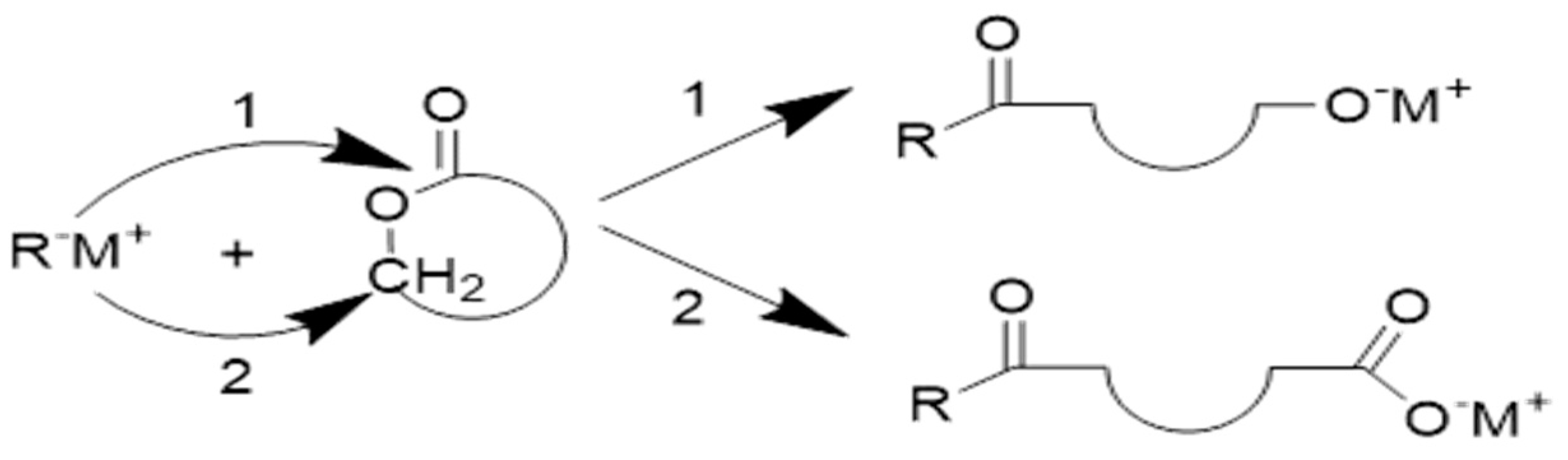
3.2.1.1. Coordination–Insertion Mechanism
3.2.1.2. Activated Monomer and Activated Chain Mechanisms
3.2.1.3. Nucleophilic Activation of the Polymer Chain End
3.3. Catalytic Systems of ROP
3.3.1. Cationic Catalysts
3.3.2. Metal Catalysts
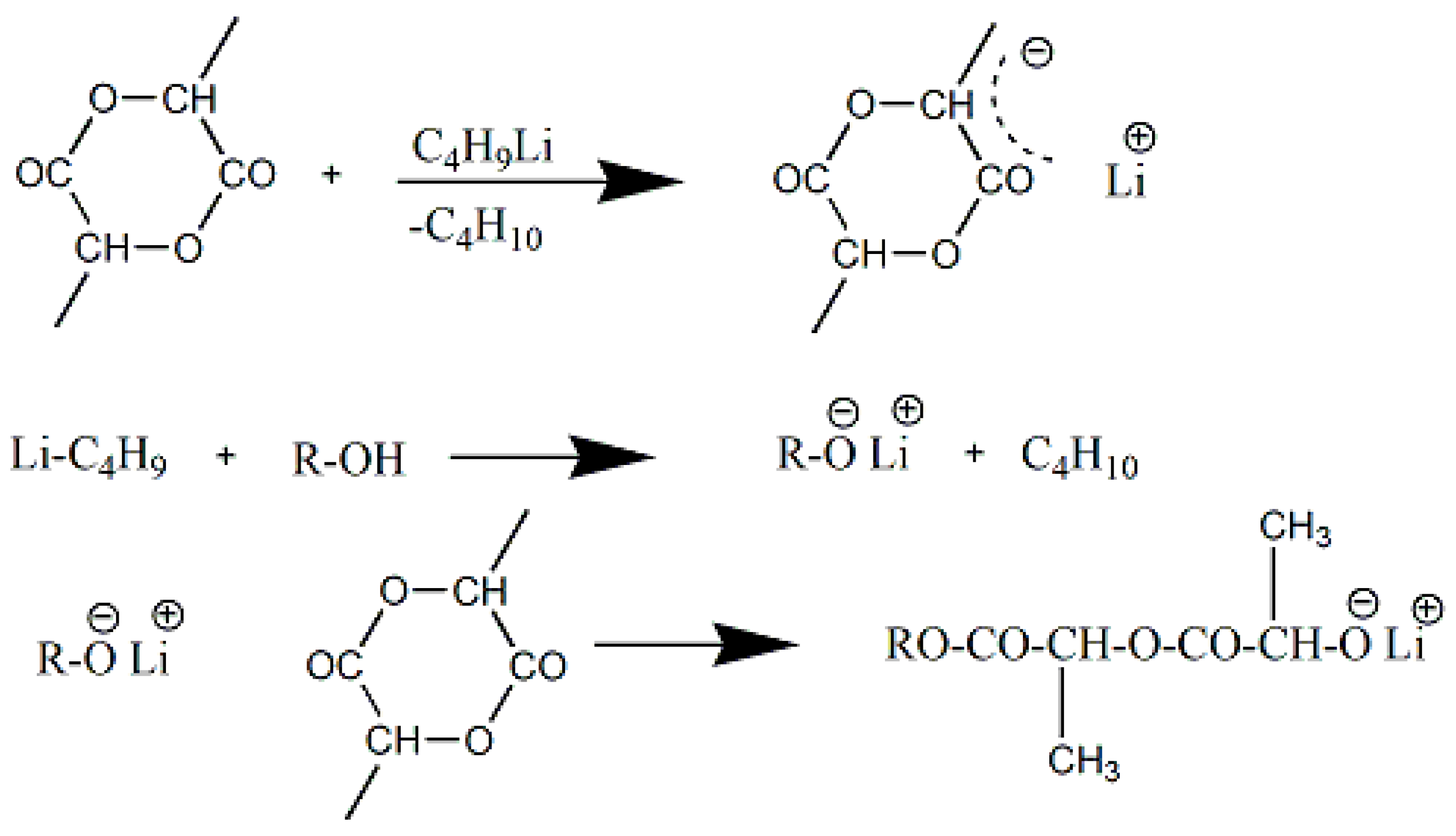
3.3.3. Anionic ROP
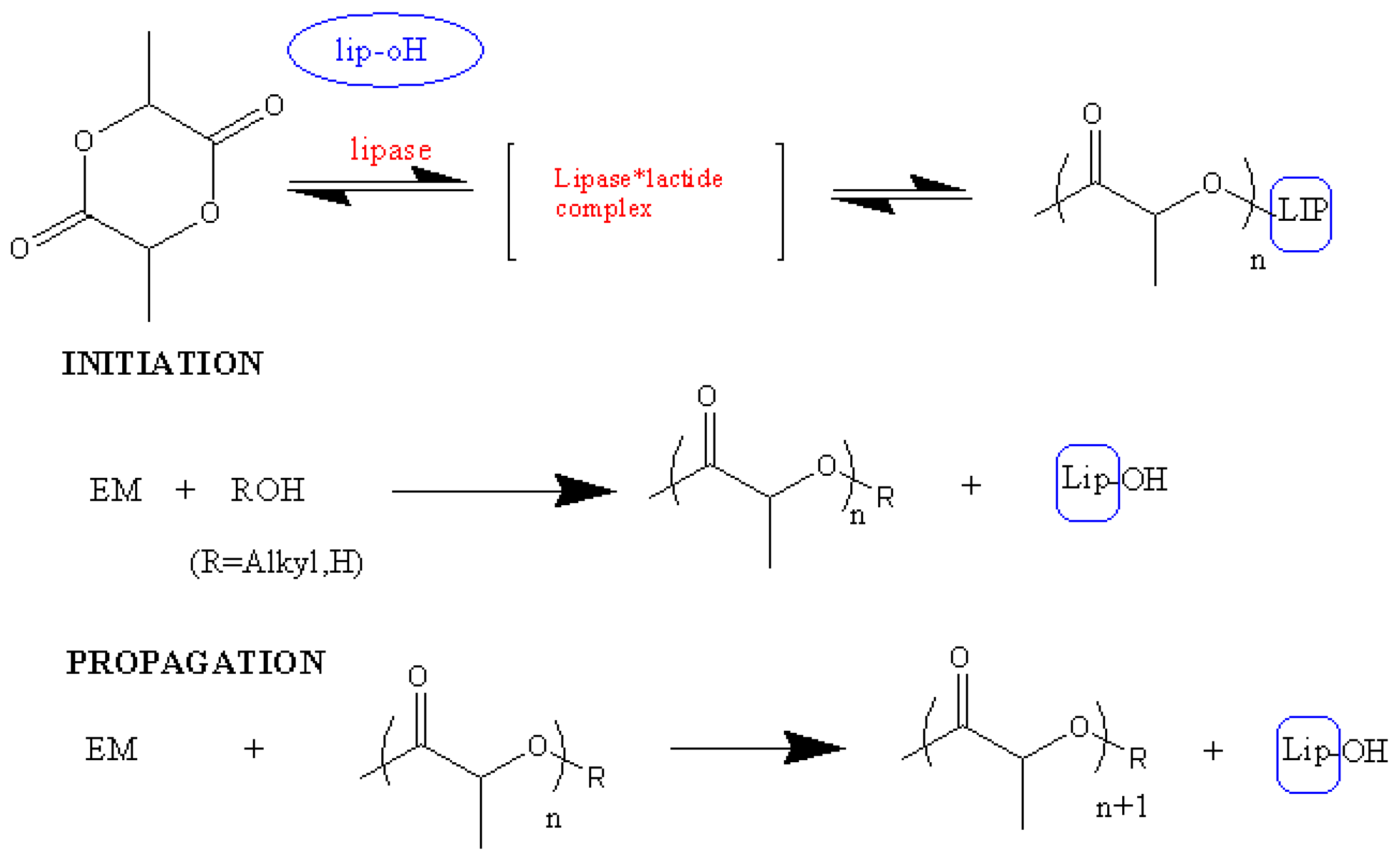
3.4. Enzymatic ROP
4. Molecular Weight Increase of PLA
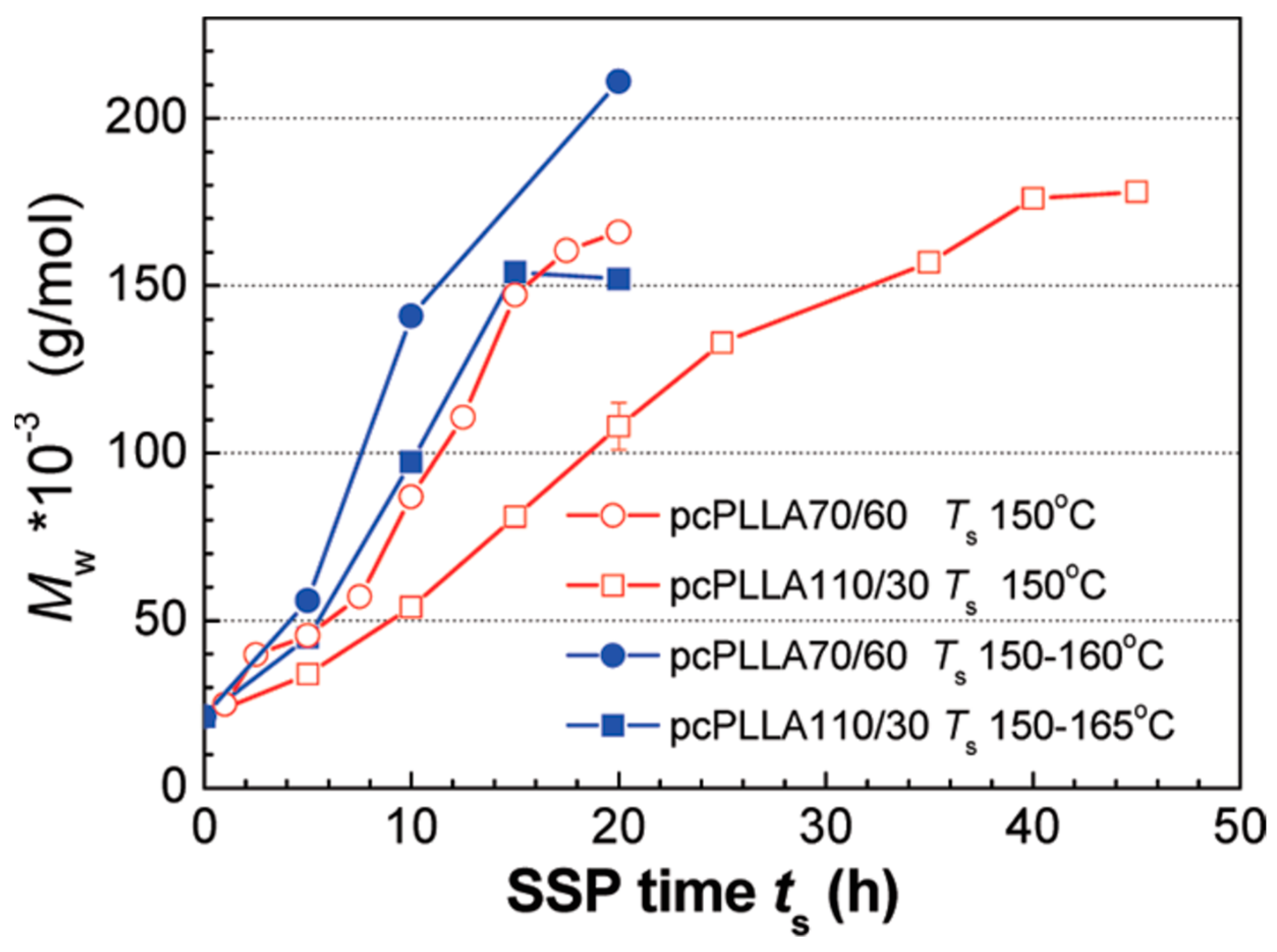
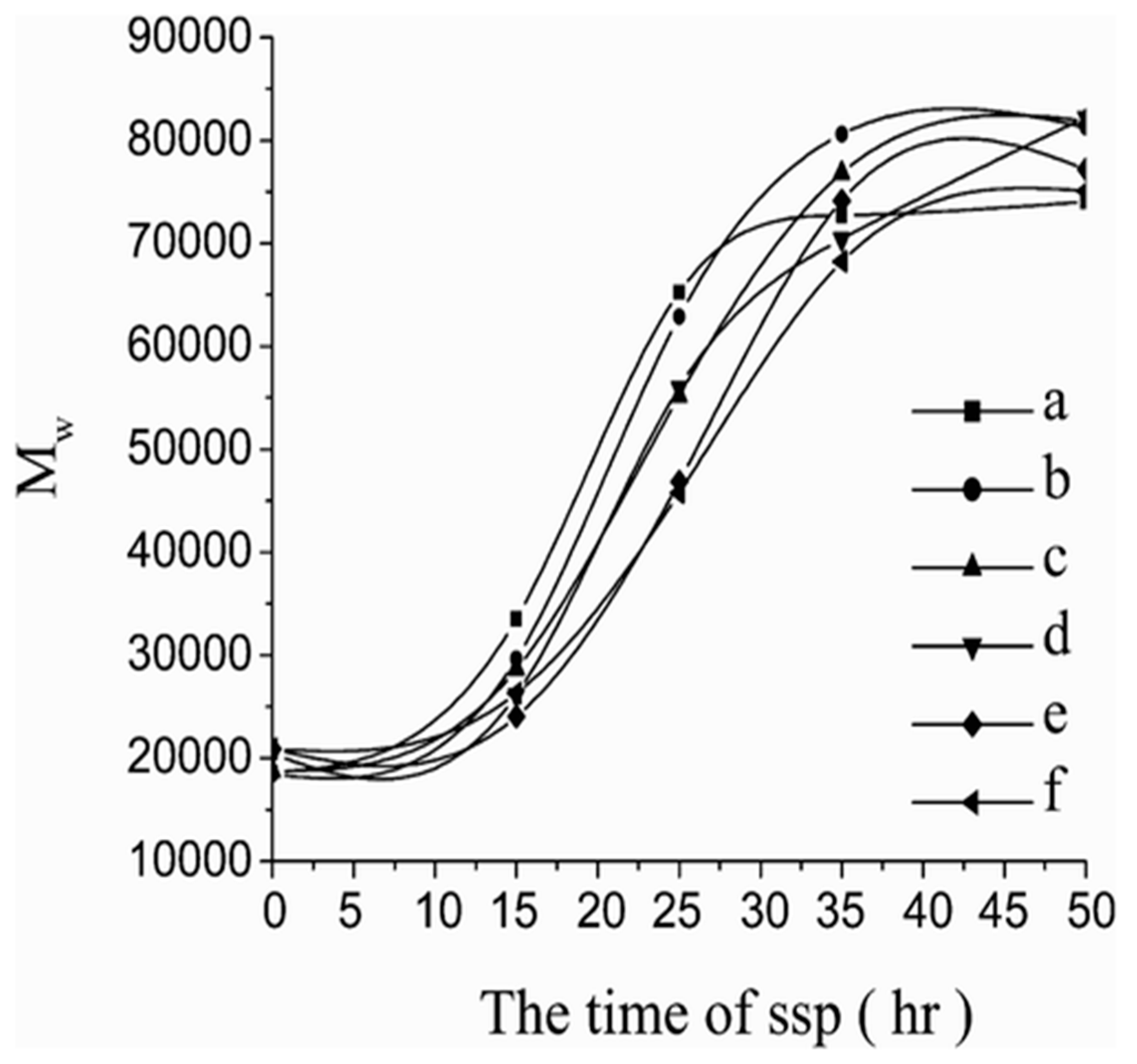
4.1. Solid State Polycondensation
4.2. Chain Extenders
5. PLA Applications
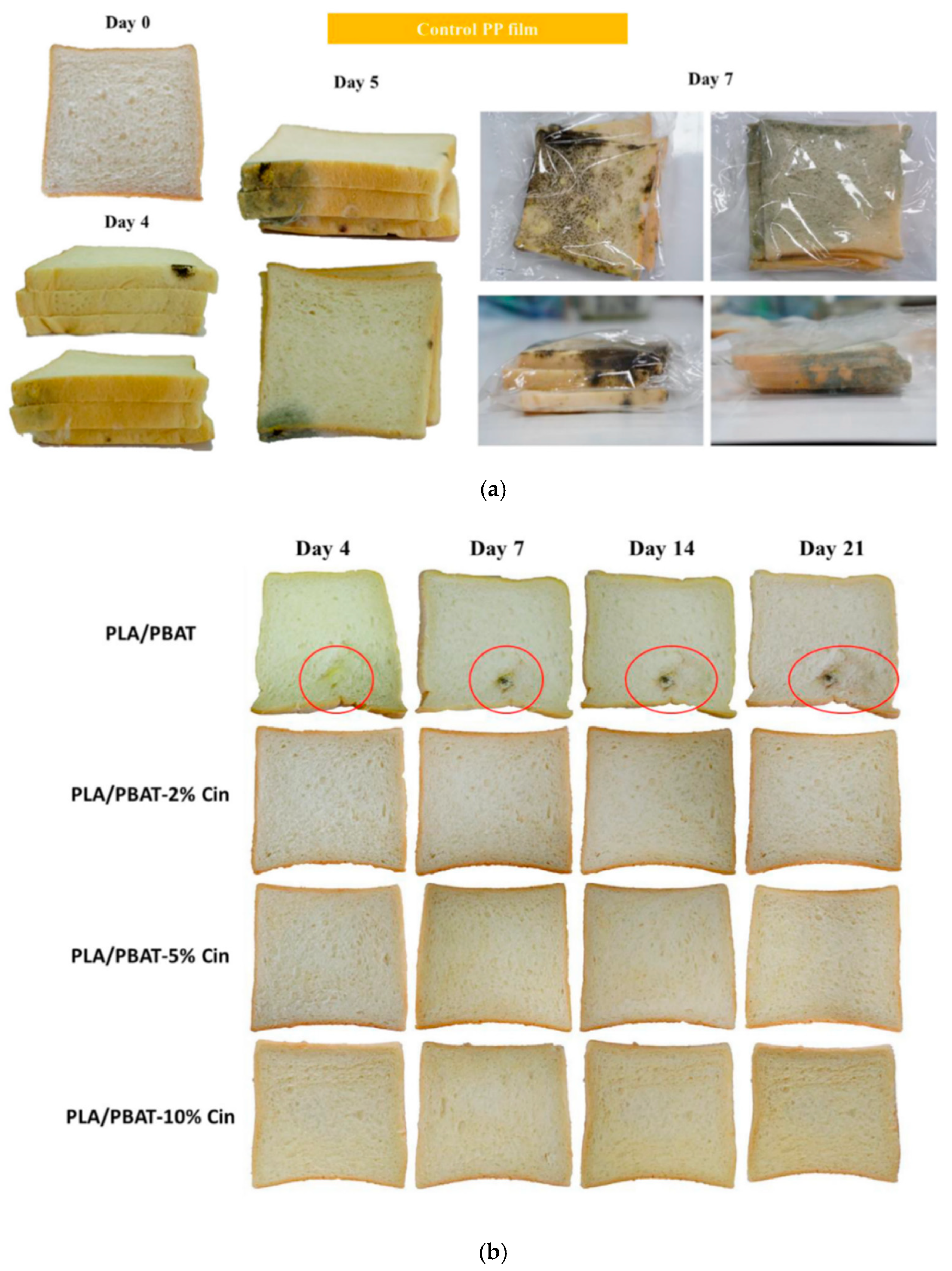
5.1. PLA for Packaging
5.2. Single Use Products
5.3. PLA for Textiles
5.4. PLA for Automotive
5.5. Agricultural Uses of PLA
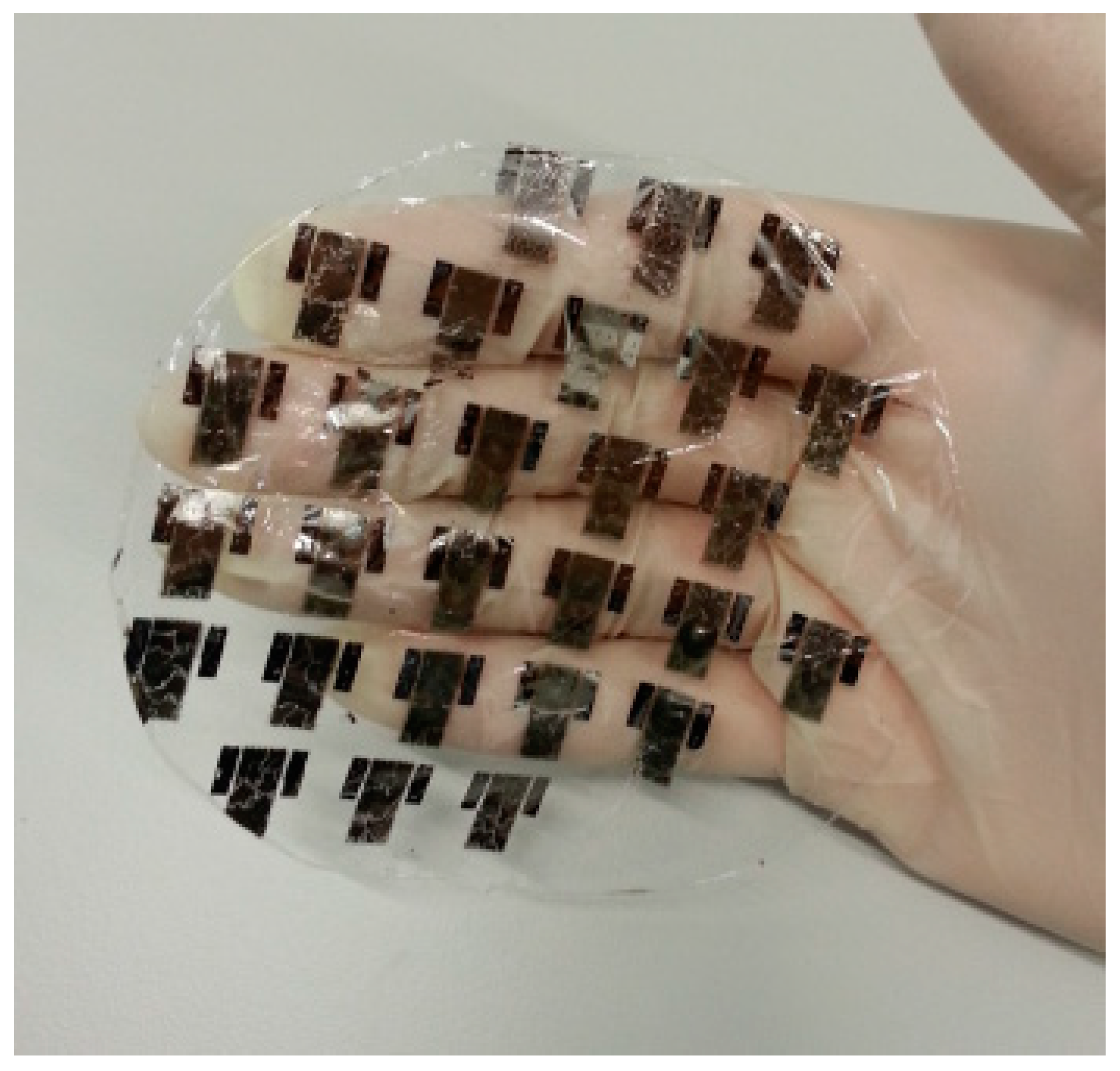
5.6. PLA for Electronic Devices
5.7. PLA for Construction
5.8. Biomedical Applications
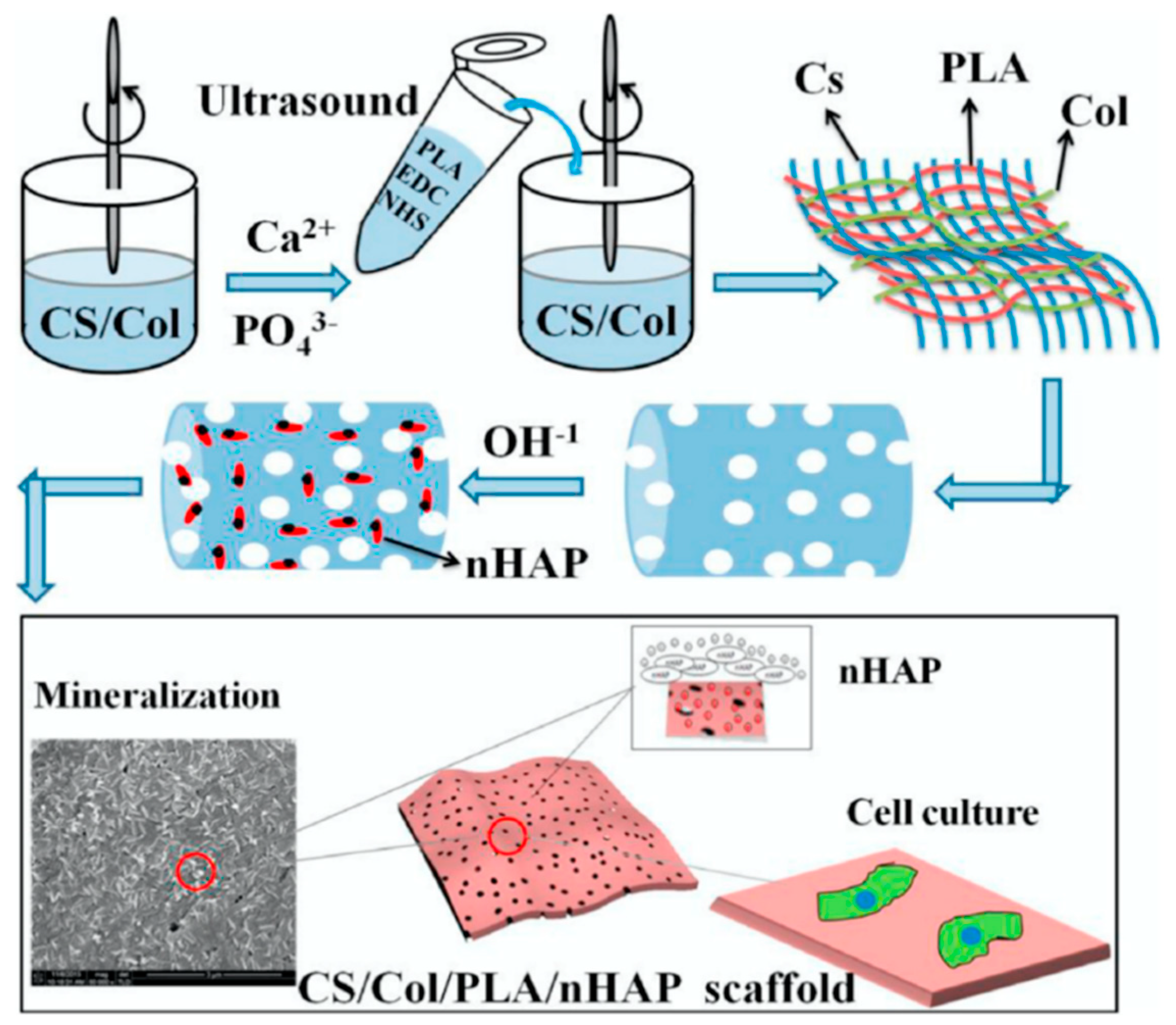
5.8.1. PLA in Bone Tissue Engineering
5.8.2. PLA for Blood Vessels and Organs
5.8.3. PLA for Skin Regeneration
5.9. Medical Applications
5.9.1. PLA for Tumor-Targeting
5.9.2. PLA for Drug Delivery
6. Technical Challenges, Limitations and Future Perspectives for Sustainable PLA Production
6.1. Technical Challenges
6.2. Limitations for Commercial Applications
6.3. Future Perspectives
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References and Note
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, use, and fate of all plastics ever made—Supplementary Information. Sci. Adv. 2017, 3, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Chinthapalli, R.; Skoczinski, P.; Carus, M.; Baltus, W.; De Guzman, D.; Käb, H.; Raschka, A.; Ravenstijn, J. Biobased building blocks and polymers—Global capacities, production and trends, 2018–2023. Ind. Biotechnol. 2019, 15, 237–241. [Google Scholar] [CrossRef]
- Pappa, A.; Papadimitriou-Tsantarliotou, A.; Kaloyianni, M.; Kastrinaki, G.; Dailianis, S.; Lambropoulou, D.A.; Christodoulou, E.; Kyzas, G.Z.; Bikiaris, D.N. Insights into the toxicity of biomaterials microparticles with a combination of cellular and oxidative biomarkers. J. Hazard. Mater. 2021, 413, 125335. [Google Scholar] [CrossRef]
- Toussaint, B.; Raffael, B.; Angers-Loustau, A.; Gilliland, D.; Kestens, V.; Petrillo, M.; Rio-Echevarria, I.M.; van den Eede, G. Review of micro- and nanoplastic contamination in the food chain. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2019, 36, 639–673. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Xu, E.G.; Li, J.; Chen, Q.; Ma, L.; Zeng, E.Y.; Shi, H. A review of microplastics in table salt, drinking water, and air: Direct human exposure. Environ. Sci. Technol. 2020, 54, 3740–3751. [Google Scholar] [CrossRef] [PubMed]
- Ganesh Saratale, R.; Cho, S.K.; Dattatraya Saratale, G.; Kadam, A.A.; Ghodake, G.S.; Kumar, M.; Naresh Bharagava, R.; Kumar, G.; Su Kim, D.; Mulla, S.I.; et al. A comprehensive overview and recent advances on polyhydroxyalkanoates (PHA) production using various organic waste streams. Bioresour. Technol. 2021, 325, 124685. [Google Scholar] [CrossRef] [PubMed]
- Babu, R.P.; O’Connor, K.; Seeram, R. Current progress on bio-based polymers and their future trends. Prog. Biomater. 2013, 2, 8. [Google Scholar] [CrossRef] [Green Version]
- Market—European Bioplastics e. 2019. Available online: https://www.european-bioplastics.org/market/ (accessed on 27 April 2021).
- Jem, K.J.; Tan, B. The development and challenges of poly (lactic acid) and poly (glycolic acid). Adv. Ind. Eng. Polym. Res. 2020, 3, 60–70. [Google Scholar] [CrossRef]
- Nakajima, H.; Dijkstra, P.; Loos, K. The recent developments in biobased polymers toward general and engineering applications: Polymers that are upgraded from biodegradable polymers, analogous to petroleum-derived polymers, and newly developed. Polymers 2017, 9, 523. [Google Scholar] [CrossRef]
- Höhnemann, T.; Steinmann, M.; Schindler, S.; Hoss, M.; König, S.; Ota, A.; Dauner, M.; Buchmeiser, M.R. Poly(Ethylene furanoate) along its life-cycle from a polycondensation approach to high-performance yarn and its recyclate. Materials 2021, 14, 1044. [Google Scholar] [CrossRef]
- Loos, K.; Zhang, R.; Pereira, I.; Agostinho, B.; Hu, H.; Maniar, D.; Sbirrazzuoli, N.; Silvestre, A.J.D.; Guigo, N.; Sousa, A.F. A Perspective on PEF Synthesis, Properties, and End-Life. Front. Chem. 2020, 8, 1–18. [Google Scholar] [CrossRef]
- Reichert, C.L.; Bugnicourt, E.; Coltelli, M.B.; Cinelli, P.; Lazzeri, A.; Canesi, I.; Braca, F.; Martínez, B.M.; Alonso, R.; Agostinis, L.; et al. Bio-based packaging: Materials, modifications, industrial applications and sustainability. Polymers 2020, 12, 1588. [Google Scholar] [CrossRef]
- Achmad, F.; Yamane, K.; Quan, S.; Kokugan, T. Synthesis of polylactic acid by direct polycondensation under vacuum without catalysts, solvents and initiators. Chem. Eng. J. 2009, 151, 342–350. [Google Scholar] [CrossRef]
- Maharana, T.; Mohanty, B.; Negi, Y.S. Melt-solid polycondensation of lactic acid and its biodegradability. Prog. Polym. Sci. 2009, 34, 99–124. [Google Scholar] [CrossRef]
- Pretula, J.; Slomkowski, S.; Penczek, S. Polylactides—Methods of synthesis and characterization. Adv. Drug Deliv. Rev. 2016, 107, 3–16. [Google Scholar] [CrossRef] [PubMed]
- Hamad, K.; Kaseem, M.; Yang, H.W.; Deri, F.; Ko, Y.G. Properties and medical applications of polylactic acid: A review. Express Polym. Lett. 2015, 9, 435–455. [Google Scholar] [CrossRef]
- DeStefano, V.; Khan, S.; Tabada, A. Applications of PLA in modern medicine. Eng. Regen. 2020, 1, 76–87. [Google Scholar] [CrossRef]
- Luo, F.; Fortenberry, A.; Ren, J.; Qiang, Z. Recent progress in enhancing poly(lactic acid) stereocomplex formation for material property improvement. Front. Chem. 2020, 8, 688. [Google Scholar] [CrossRef] [PubMed]
- Jin, F.L.; Hu, R.R.; Park, S.J. Improvement of thermal behaviors of biodegradable poly(lactic acid) polymer: A review. Compos. Part B Eng. 2019, 164, 287–296. [Google Scholar] [CrossRef]
- Zaaba, N.F.; Jaafar, M. A review on degradation mechanisms of polylactic acid: Hydrolytic, photodegradative, microbial, and enzymatic degradation. Polym. Eng. Sci. 2020, 60, 2061–2075. [Google Scholar] [CrossRef]
- Ren, J. Biodegradable Poly(Lactic Acid): Synthesis, Modification, Processing; Springer: Heidelberg, Germany; Dordrecht, The Netherlands; London, UK; New York, NY, USA; Beijing, China, 2010; ISBN 9787302236016. [Google Scholar]
- Abedi, E.; Hashemi, S.M.B. Lactic acid production—Producing microorganisms and substrates sources-state of art. Heliyon 2020, 6, e04974. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Lactic acid production from lignocellulose-derived sugars using lactic acid bacteria: Overview and limits. J. Biotechnol. 2011, 156, 286–301. [Google Scholar] [CrossRef]
- Abdel-Rahman, M.A.; Tashiro, Y.; Sonomoto, K. Recent advances in lactic acid production by microbial fermentation processes. Biotechnol. Adv. 2013, 31, 877–902. [Google Scholar] [CrossRef]
- Helen Shiphrah, V.; Sahu, S.; Ranjan Thakur, A.; Ray Chaudhuri, S. Screening of bacteria for lactic acid production from whey water. Am. J. Biochem. Biotechnol. 2013, 9, 118–123. [Google Scholar] [CrossRef]
- Nduko, J.M.; Taguchi, S. Microbial production of biodegradable lactate-based polymers and oligomeric building blocks from renewable and waste resources. Front. Bioeng. Biotechnol. 2021, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Hong, C.H.; Kim, S.H.; Seo, J.-Y.; Han, D.S. Development of four unit processes for biobased PLA manufacturing. ISRN Polym. Sci. 2012, 2012, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, N.; Roychoudhury, P.K.; Srivastava, A. L (+) lactic acid fermentation and its product polymerization. Electron. J. Biotechnol. 2004, 7. [Google Scholar] [CrossRef]
- Qi, B.; Risheng, Y. L-lactic acid production from Lactobacillus casei by solid state fermentation using rice straw. BioResources 2007, 2, 419–429. [Google Scholar] [CrossRef]
- Park, J.; Cho, H.; Hwang, D.; Kim, S.; Moon, I.; Kim, M. Design of a novel process for continuous lactide synthesis from lactic acid. Ind. Eng. Chem. Res. 2018, 57, 11955–11962. [Google Scholar] [CrossRef]
- Ehsani, M.; Khodabakhshi, K.; Asgari, M. Lactide synthesis optimization: Investigation of the temperature, catalyst and pressure effects. E-Polymers 2014, 14, 353–361. [Google Scholar] [CrossRef]
- VanWouwe, P.; Dusselier, M.; Vanleeuw, E.; Sels, B. Lactide synthesis and chirality control for polylactic acid production. ChemSusChem 2016, 9, 907–921. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L. Synthesis and biological application of polylactic acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.T.; Auras, R.; Rubino, M. Processing technologies for poly(lactic acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Nagahata, R.; Sano, D.; Suzuki, H.; Takeuchi, K. Microwave-assisted single-step synthesis of poly(lactic acid) by direct polycondensation of lactic acid. Macromol. Rapid Commun. 2007, 28, 437–442. [Google Scholar] [CrossRef]
- Montané, X.; Montornes, J.M.; Nogalska, A.; Olkiewicz, M.; Giamberini, M.; Garcia-Valls, R.; Badia-Fabregat, M.; Jubany, I.; Tylkowski, B. Synthesis and synthetic mechanism of Polylactic acid. Phys. Sci. Rev. 2020, 5, 1–11. [Google Scholar] [CrossRef]
- Shahrul Nizan Shikh Zahari, S.M.; Mansor, M.H.; Azman, H.H.; Rosli, D. Uncatalysed Polycondensation of Lactic Acid to Polylactic Acid under Microwave Irradiation: Effect of Microwave Power. J. Phys. Conf. Ser. 2020, 1551. [Google Scholar] [CrossRef]
- Hu, Y.; Daoud, W.A.; Cheuk, K.K.L.; Lin, C.S.K. Newly developed techniques on polycondensation, ring-opening polymerization and polymer modification: Focus on poly(lactic acid). Materials 2016, 9, 133. [Google Scholar] [CrossRef] [Green Version]
- Moon, S.I.; Lee, C.W.; Miyamoto, M.; Kimura, Y. Melt polycondensation of L-lactic acid with Sn(II) catalysts activated by various proton acids: A direct manufacturing route to high molecular weight poly(L-lactic acid). J. Polym. Sci. Part A Polym. Chem. 2000, 38, 1673–1679. [Google Scholar] [CrossRef]
- Peng, Q.; Mahmood, K.; Wu, Y.; Liu, Z.; Wei, L.; Yuan, H.; Yang, R. Effective binary catalysts of BrØnsted acidic ionic liquids and stannous chloride dihydrate for melt polycondensation of L-lactic acid. Mol. Catal. 2017, 434, 140–145. [Google Scholar] [CrossRef]
- Barleany, D.R.; Prasetya, B.; Andiyani, T. Polylactic acid synthesis via direct polycondensation method using candida rugosa lipase catalyst. World Chem. Eng. J. 2017, 1, 70–74. [Google Scholar]
- Singh, S.K.; Anthony, P.; Chowdhury, A. High molecular weight poly(lactic acid) synthesized with apposite catalytic combination and longer time. Orient. J. Chem. 2018, 34, 1984–1990. [Google Scholar] [CrossRef]
- Chen, G.X.; Kim, H.S.; Kim, E.S.; Yoon, J.S. Synthesis of high-molecular-weight poly(l-lactic acid) through the direct condensation polymerization of l-lactic acid in bulk state. Eur. Polym. J. 2006, 42, 468–472. [Google Scholar] [CrossRef]
- Eğri, Ö. Use of microperlite in direct polymerization of lactic acid. Int. J. Polym. Anal. Charact. 2019, 24, 142–149. [Google Scholar] [CrossRef]
- Horváth, T.; Szabó, T.J.; Marossy, K. Molar mass determination of microwave initiated polycondensation produced PLLA by capillary viscometry method. J. Phys. Conf. Ser. 2020, 1527. [Google Scholar] [CrossRef]
- Maryanty, Y.; Hadiantoro, S.; Widjajanti, K.; Putri, D.I.K.; Nikmah, L. Poly lactic acid (pla) development using lactic acid product from rice straw fermentation with azeotropic polycondensation process. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1053, 012043. [Google Scholar] [CrossRef]
- Williams, C.K. Synthesis of functionalized biodegradable polyesters. Chem. Soc. Rev. 2007, 36, 1573–1580. [Google Scholar] [CrossRef]
- Peng, Y.; Liu, Q.J.; He, T.; Ye, K.; Yao, X.; Ding, J. Degradation rate affords a dynamic cue to regulate stem cells beyond varied matrix stiffness. Biomaterials 2018, 178, 467–480. [Google Scholar] [CrossRef]
- Liu, Q.; Zheng, S.; Ye, K.; He, J.; Shen, Y.; Cui, S.; Huang, J.; Gu, Y.; Ding, J. Cell migration regulated by RGD nanospacing and enhanced under moderate cell adhesion on biomaterials. Biomaterials 2020, 263, 120327. [Google Scholar] [CrossRef]
- Xu, H.; Ma, B.; Jiang, J.; Xiao, S.; Peng, R.; Zhuang, W.; Li, G.; Wang, Y. Integrated prodrug micelles with two-photon bioimaging and pH-triggered drug delivery for cancer theranostics. Regen. Biomater. 2020, 7, 171–180. [Google Scholar] [CrossRef] [Green Version]
- Dove, A.P. Controlled ring-opening polymerisation of cyclic esters: Polymer blocks in self-assembled nanostructures. Chem. Commun. 2008, 6446–6470. [Google Scholar] [CrossRef] [PubMed]
- Kowalski, A.; Libiszowski, J.; Majerska, K.; Duda, A.; Penczek, S. Kinetics and mechanism of ε-caprolactone and l,l-lactide polymerization coinitiated with zinc octoate or aluminum acetylacetonate: The next proofs for the general alkoxide mechanism and synthetic applications. Polymer (Guildf) 2007, 48, 3952–3960. [Google Scholar] [CrossRef]
- Duan, R.; Qu, Z.; Pang, X.; Zhang, Y.; Sun, Z.; Zhang, H.; Bian, X.; Chen, X. Ring-Opening Polymerization of Lactide Catalyzed by Bimetallic Salen-Type Titanium Complexes. Chin. J. Chem. 2017, 35, 640–644. [Google Scholar] [CrossRef]
- Byers, J.A.; Biernesser, A.B.; Delle Chiaie, K.R.; Kaur, A.; Kehl, J.A. Catalytic systems for the production of poly(lactic acid). Adv. Polym. Sci. 2018, 279, 67–118. [Google Scholar] [CrossRef]
- Berl, M.; Kricheldorf, H.R.; Scharnagl, N. Polymerisation Mechanism of Metal Alkoxide Initiated Polymerisations of Lactide and Various Lactones. Macromolecules 1988, 21, 286. [Google Scholar]
- Dubois, P.; Jacobs, C.; Jérôme, R.; Teyssté, P. Macromolecular engineering of polylactones and polylactides. 4. Mechanism and kinetics of lactidehomopolymerization by aluminum isopropoxide. Macromolecules 1991, 24, 2266–2270. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura. PLA Synthesis and Polymerization; Royal Society of Chemistry: London, UK, 2014; ISBN 9781782624806. [Google Scholar]
- Baśko, M.; Kubisa, P. Cationic polymerization of L,L-lactide. J. Polym. Sci. Part A Polym. Chem. 2010, 48, 2650–2658. [Google Scholar] [CrossRef]
- Matsumura, S.; Hlil, A.R.; Lepiller, C.; Gaudet, J.; Guay, D.; Shi, Z.; Holdcroft, S.; Hay, A.S. Stability and utility of pyridyl disulfide functionality in RAFT and conventional radical polymerizations. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 7207–7224. [Google Scholar] [CrossRef]
- Paquin, F.; Rivnay, J.; Salleo, A.; Stingelin, N.; Silva, C. Multi-phase semicrystalline microstructures drive exciton dissociation in neat plastic semiconductors. J. Mater. Chem. C 2015, 3, 10715–10722. [Google Scholar] [CrossRef] [Green Version]
- Mandal, M.; Ramkumar, V.; Chakraborty, D. Salen complexes of zirconium and hafnium: Synthesis, structural characterization and polymerization studies. Polym. Chem. 2019, 10, 3444–3460. [Google Scholar] [CrossRef]
- Mandal, M.; Chakraborty, D. Co2O3 and MnO2 as inexpensive catalysts for the ring-opening polymerization of cyclic esters. J. Polym. Res. 2021, 28. [Google Scholar] [CrossRef]
- Chuma, A.; Horn, H.W.; Swope, W.C.; Pratt, R.C.; Zhang, L.; Lohmeijer, B.G.G.; Wade, C.G.; Waymouth, R.M.; Hedrick, J.L.; Rice, J.E. The reaction mechanism for the organocatalytic ring-opening polymerization of L-lactide using a guanidine-based catalyst: Hydrogen-bonded or covalently bound? J. Am. Chem. Soc. 2008, 130, 6749–6754. [Google Scholar] [CrossRef]
- Toshikj, N.; Robin, J.J.; Blanquer, S. A simple and general approach for the synthesis of biodegradable triblock copolymers by organocatalytic ROP from poly(lactide) macroinitiators. Eur. Polym. J. 2020, 127, 109599. [Google Scholar] [CrossRef]
- Coulembier, O.; Lohmeijer, B.G.G.; Dove, A.P.; Pratt, R.C.; Mespouille, L.; Culkin, D.A.; Benight, S.J.; Dubois, P.; Waymouth, R.M.; Hedrick, J.L. Alcohol adducts of N-heterocyclic carbenes: Latent catalysts for the thermally-controlled living polymerization of cyclic esters. Macromolecules 2006, 39, 5617–5628. [Google Scholar] [CrossRef]
- Coulembier, O.; Dove, A.P.; Pratt, R.C.; Sentman, A.C.; Culkin, D.A.; Mespouille, L.; Dubois, P.; Waymouth, R.M.; Hedrick, J.L. Latent, thermally activated organic catalysts for the on-demand living polymerization of lactide. Angew. Chemie Int. Ed. 2005, 44, 4964–4968. [Google Scholar] [CrossRef] [PubMed]
- Nuyken, O.; Pask, S.D. Ring-opening polymerization-An introductory review. Polymers 2013, 5, 361–403. [Google Scholar] [CrossRef] [Green Version]
- Bourissou, D.; Moebs-sanchez, S.; Martı, B. Recent advances in the controlled preparation of poly (a-hydroxy acids): Metal-free catalysts and new monomers. C. R. Chimie 2007, 10, 10. [Google Scholar] [CrossRef]
- Sungyeap Hong, C.L. An Overview of the Synthesis and Synthetic Mechanism of Poly (Lactic acid). Mod. Chem. Appl. 2014, 2. [Google Scholar] [CrossRef] [Green Version]
- Zaremba, L.S.; Smoleński, W.H. Optimal portfolio choice under a liability constraint. Ann. Oper. Res. 2000, 97, 131–141. [Google Scholar] [CrossRef]
- Kubisa, P. Activated monomer mechanism in the cationic polymerization of cyclic ethers. Makromol. Chemie Macromol. Symp. 1988, 13–14, 203–210. [Google Scholar] [CrossRef]
- Bourissou, D.; Martin-Vaca, B.; Dumitrescu, A.; Graullier, M.; Lacombe, F. Controlled cationic polymerization of lactide. Macromolecules 2005, 38, 9993–9998. [Google Scholar] [CrossRef]
- Metkar, S.; Sathe, V.; Rahman, I.; Idage, B.; Idage, S. Ring opening polymerization of lactide: Kinetics and modeling. Chem. Eng. Commun. 2019, 206, 1159–1167. [Google Scholar] [CrossRef]
- Karidi, K.; Pladis, P.; Kiparissides, C. A theoretical and experimental kinetic investigation of the ROP of L,L-lactide in the presence of polyalcohols. Macromol. Symp. 2013, 333, 206–215. [Google Scholar] [CrossRef]
- Medina, D.A.; Contreras, J.M.; López-Carrasquero, F.J.; Cardozo, E.J.; Contreras, R.R. Use of samarium(III)–amino acid complexes as initiators of ring-opening polymerization of cyclic esters. Polym. Bull. 2018, 75, 1253–1263. [Google Scholar] [CrossRef]
- Kricheldorf, H.R.; Boettcher, C. Polylactones 26†. Lithium alkoxide-initiated polymerizations of L-lactide. In Die Makromolekulare Chemie; Wiley: Hoboken, NJ, USA, 1993; Volume 1669, pp. 1665–1669. [Google Scholar]
- Stridsberg, K.M.; Ryner, M.; Albertsson, A.C. Controlled ring-opening polymerization: Polymers with designed macromolecular architecture. In Degradable Aliphatic Polyesters; Springer: Berlin/Heidelberg, Germany, 2002; ISBN 9171705228. [Google Scholar]
- Zhu, Z.; Deng, X.; Xiong, C. Anionic ring-opening polymerization of D,L-lactide. Indian J. Chem. Sect. B Org. Med. Chem. 2001, 40, 108–112. [Google Scholar]
- Zhao, H.; Nathaniel, G.A.; Merenini, P.C. Enzymatic ring-opening polymerization (ROP) of lactides and lactone in ionic liquids and organic solvents: Digging the controlling factors. RSC Adv. 2017, 7, 48639–48648. [Google Scholar] [CrossRef] [Green Version]
- Engel, J.; Cordellier, A.; Huang, L.; Kara, S. Enzymatic ring-opening polymerization of lactones: Traditional approaches and alternative strategies. ChemCatChem 2019, 11, 4983–4997. [Google Scholar] [CrossRef]
- Omay, D.; Guvenilir, Y. Synthesis and characterization of poly(d,l-lactic acid) via enzymatic ring opening polymerization by using free and immobilized lipase. Biocatal. Biotransform. 2013, 31, 132–140. [Google Scholar] [CrossRef]
- Whulanza, Y.; Rahman, S.F.; Suyono, E.A.; Yohda, M.; Gozan, M. Use of Candida rugosa lipase as a biocatalyst for L-lactide ring-opening polymerization and polylactic acid production. Biocatal. Agric. Biotechnol. 2018, 16, 683–691. [Google Scholar] [CrossRef]
- Cheng, Y.; Deng, S.; Chen, P.; Ruan, R. Polylactic acid (PLA) synthesis and modifications: A review. Front. Chem. China 2009, 4, 259–264. [Google Scholar] [CrossRef]
- Vouyiouka, S.; Theodoulou, P.; Symeonidou, A.; Papaspyrides, C.D.; Pfaendner, R. Solid state polymerization of poly(lactic acid): Some fundamental parameters. Polym. Degrad. Stab. 2013, 98, 2473–2481. [Google Scholar] [CrossRef]
- Beltrán, F.R.; Climent-Pascual, E.; de la Orden, M.U.; Martínez Urreaga, J. Effect of solid-state polymerization on the structure and properties of mechanically recycled poly(lactic acid). Polym. Degrad. Stab. 2020, 171. [Google Scholar] [CrossRef]
- Woong Kim, K.; Woo, S.Ii. Synthesis of high-molecular-weight poly(L-lactic acid) by direct polycondensation. Macromol. Chem. Phys. 2002, 203, 2245–2250. [Google Scholar] [CrossRef]
- Takenaka, M.; Kimura, Y.; Ohara, H. Molecular weight increase driven by evolution of crystal structure in the process of solid-state polycondensation of poly(L-lactic acid). Polymer (Guildf) 2017, 126, 133–140. [Google Scholar] [CrossRef]
- Peng, B.; Hou, H.; Song, F.; Wu, L. Synthesis of high molecular weight poly(l -lactic acid) via melt/solid state polycondensation. II. Effect of precrystallization on solid state polycondensation. Ind. Eng. Chem. Res. 2012, 51, 5190–5196. [Google Scholar] [CrossRef]
- Xu, H.; Luo, M.; Yu, M.; Teng, C.; Xie, S. The effect of crystallization on the solid state polycondensation of poly(L-lactic acid). J. Macromol. Sci. Part B Phys. 2006, 45 B, 681–687. [Google Scholar] [CrossRef]
- Kucharczyk, P.; Poljansek, I.; Sedlarik, V. The effect of various catalytic systems on solid-state polymerization of poly-(L-lactic acid). J. Macromol. Sci. Part A Pure Appl. Chem. 2012, 49, 795–805. [Google Scholar] [CrossRef]
- Vimal Katiyar, H.N. Solid State Polymerization of Poly(L-Lactide): Multiple-Fold Increase in Molecular Weight via an Efficient Catalyst System. Polym. Eng. Sci. 2011, 51, 2078–2084. [Google Scholar] [CrossRef]
- Fukushima, K.; Furuhashi, Y.; Sogo, K.; Miura, S.; Kimura, Y. Stereoblock poly(lactic acid): Synthesis via solid-state polycondensation of a stereocomplexed mixture of poly(L-lactic acid) and poly(D-lactic acid). Macromol. Biosci. 2005, 5, 21–29. [Google Scholar] [CrossRef] [PubMed]
- Fukushima, K.; Kimura, Y. An Efficient Solid-State Polycondensation Method for Synthesizing Stereocomplexed Poly(Lactic Acid)s with High Molecular Weight. J. Polym. Sci. Part A Polym. Chem. 2008, 46, 3714–3722. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Chain extension of polyesters PET and PBT with N,N’-Bis(glycidy1 ester) Pyromellitimides. I. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1705–1714. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Thermomechanical analysis of chain-extended PET and PBT. J. Appl. Polym. Sci. 1996, 60, 55–61. [Google Scholar] [CrossRef]
- Xanthos, M.; Young, M.W.; Karayannidis, G.P.; Bikiaris, D.N. Reactive modification of poly(ethylene terephthalate) with polyepoxides. Polym. Eng. Sci. 2001, 41, 643–655. [Google Scholar] [CrossRef]
- Bikiaris, D.N.; Karayannidis, G.P. Chain extension of polyesters PET and PBT with two new diimidodiepoxides. II. J. Polym. Sci. Part A Polym. Chem. 1996, 34, 1337–1342. [Google Scholar] [CrossRef]
- Meng, Q.; Heuzey, M.C.; Carreau, P.J. Control of thermal degradation of polylactide/clay nanocomposites during melt processing by chain extension reaction. Polym. Degrad. Stab. 2012, 97, 2010–2020. [Google Scholar] [CrossRef]
- Standau, T.; Zhao, C.; Castellón, S.M.; Bonten, C.; Altstädt, V. Chemical modification and foam processing of polylactide (PLA). Polymers 2019, 11, 306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaszkiewicz, A.; Bledzki, A.K.; van der Meer, R.; Franciszczak, P.; Meljon, A. How does a chain-extended polylactide behave?: A comprehensive analysis of the material, structural and mechanical properties. Polym. Bull. 2014, 71, 1675–1690. [Google Scholar] [CrossRef] [Green Version]
- Elhassan, A.S.M.; Saeed, H.A.M.; Eltahir, Y.A.; Xia, Y.M.; Wang, Y.P. Modification of PLA with chain extender. Appl. Mech. Mater. 2014, 716–717, 44–47. [Google Scholar] [CrossRef]
- Li, Y.; Zhou, H.; Wen, B.; Chen, Y.; Wang, X. A facile and efficient method for preparing chain extended poly(lactic acid) foams with high volume expansion ratio. J. Polym. Environ. 2020, 28, 17–31. [Google Scholar] [CrossRef]
- Ludwiczak, J.; Kozlowski, M. Foaming of polylactide in the presence of chain extender. J. Polym. Environ. 2015, 23, 137–142. [Google Scholar] [CrossRef] [Green Version]
- Yahyaee, N.; Javadi, A.; Garmabi, H.; Khaki, A. Effect of two-step chain extension using joncryl and PMDA on the rheological properties of poly(lactic acid). Macromol. Mater. Eng. 2020, 305, 1900423. [Google Scholar] [CrossRef]
- Karkhanis, S.S.; Matuana, L.M. Extrusion blown films of poly(lactic acid) chain-extended with food grade multifunctional epoxies. Polym. Eng. Sci. 2019, 59, 2211–2219. [Google Scholar] [CrossRef]
- Quiles-Carrillo, L.; Duart, S.; Montanes, N.; Torres-Giner, S.; Balart, R. Enhancement of the mechanical and thermal properties of injection-molded polylactide parts by the addition of acrylated epoxidized soybean oil. Mater. Des. 2018, 140, 54–63. [Google Scholar] [CrossRef]
- Hao, Y.; Li, Y.; Liu, Z.; Yan, X.; Tong, Y.; Zhang, H. Thermal, mechanical and rheological properties of poly(lactic acid) chain extended with polyaryl polymethylene isocyanate. Fibers Polym. 2019, 20, 1766–1773. [Google Scholar] [CrossRef]
- Khankrua, R.; Pivsa-Art, S.; Hiroyuki, H.; Suttiruengwong, S. Effect of chain extenders on thermal and mechanical properties of poly(lactic acid) at high processing temperatures: Potential application in PLA/Polyamide 6 blend. Polym. Degrad. Stab. 2014, 108, 232–240. [Google Scholar] [CrossRef]
- Hung, C.Y.; Wang, C.C.; Chen, C.Y. Enhanced the thermal stability and crystallinity of polylactic acid (PLA) by incorporated reactive PS-b-PMMA-b-PGMA and PS-b-PGMA block copolymers as chain extenders. Polymer (Guildf) 2013, 54, 1860–1866. [Google Scholar] [CrossRef]
- Ramírez-Herrera, C.A.; Flores-Vela, A.I.; Torres-Huerta, A.M.; Domínguez-Crespo, M.A.; Palma-Ramírez, D. PLA degradation pathway obtained from direct polycondensation of 2-hydroxypropanoic acid using different chain extenders. J. Mater. Sci. 2018, 53, 10846–10871. [Google Scholar] [CrossRef]
- Jiang, Y.; Li, Z.; Song, S.; Sun, S.; Li, Q. Highly-modified polylactide transparent blends with better heat-resistance, melt strength, toughness and stiffness balance due to the compatibilization and chain extender effects of methacrylate-co-glycidyl methacrylate copolymer. J. Appl. Polym. Sci. 2021, 138, 1–12. [Google Scholar] [CrossRef]
- Zhou, M.; Zhou, P.; Xiong, P.; Qian, X.; Zheng, H. Crystallization, rheology and foam morphology of branched PLA prepared by novel type of chain extender. Macromol. Res. 2015, 23, 231–236. [Google Scholar] [CrossRef]
- Sirisinha, K.; Samana, K. Improvement of melt stability and degradation efficiency of poly (lactic acid) by using phosphite. J. Appl. Polym. Sci. 2021, 138, 1–11. [Google Scholar] [CrossRef]
- Polylactic Acid Market Size Share and Trends. Available online: www.researchandmarkets.com (accessed on 17 April 2021).
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [Green Version]
- MRFR. Polylactic Acid Market Size, Trends, Industry Growth and Research Report—Forecast to 2027; MRFR: Pune, India, 2021; 138p. [Google Scholar]
- Bioplastics & Biopolymers Market by Type (Non-Biodegradable/Bio-Based, Biodegradable), End-Use Industry (Packaging, Consumer Goods, Automotive & Transportation, Textiles, Agriculture & Horticulture), Region—Global Forecast to 2025 COVID-19 Speak to Analyst. 2020, 6454.
- Fredi, G.; Rigotti, D.; Bikiaris, D.N.; Dorigato, A. Tuning thermo-mechanical properties of poly(lactic acid) films through blending with bioderived poly(alkylene furanoate)s with different alkyl chain length for sustainable packaging. Polymer (Guildf) 2021, 218, 123527. [Google Scholar] [CrossRef]
- Süfer, Ö. Poly (Lactic Acid) Films in Food Packaging Systems. Food Sci. Nutr. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Turalija, M.; Bischof, S.; Budimir, A.; Gaan, S. Antimicrobial PLA films from environment friendly additives. Compos. Part B Eng. 2016, 102, 94–99. [Google Scholar] [CrossRef]
- Risyon, N.P.; Othman, S.H.; Basha, R.K.; Talib, R.A. Characterization of polylactic acid/halloysite nanotubes bionanocomposite films for food packaging. Food Packag. Shelf Life 2020, 23, 100450. [Google Scholar] [CrossRef]
- Zhang, L.; Huang, C.; Xu, Y.; Huang, H.; Zhao, H.; Wang, J.; Wang, S. Synthesis and characterization of antibacterial polylactic acid film incorporated with cinnamaldehyde inclusions for fruit packaging. Int. J. Biol. Macromol. 2020, 164, 4547–4555. [Google Scholar] [CrossRef]
- Ghozali, M.; Fahmiati, S.; Triwulandari, E.; Restu, W.K.; Farhan, D.; Wulansari, M.; Fatriasari, W. PLA/metal oxide biocomposites for antimicrobial packaging application. Polym. Technol. Mater. 2020, 59, 1332–1342. [Google Scholar] [CrossRef]
- Zhang, R.; Lan, W.; Ji, T.; Sameen, D.E.; Ahmed, S.; Qin, W.; Liu, Y. Development of polylactic acid/ZnO composite membranes prepared by ultrasonication and electrospinning for food packaging. Lwt 2021, 135, 110072. [Google Scholar] [CrossRef]
- Basil, C.; Blends, O.; Salmas, C.E.; Giannakas, A.E.; Baikousi, M.; Leontiou, A.; Siasou, Z.; Karakassides, M.A. Development of poly(L-Lactic acid)/chitosan/basil oil active packaging films via a melt-extrusion process using novel chitosan/basil oil blends. Processes 2021, 9, 88. [Google Scholar] [CrossRef]
- Vorawongsagul, S.; Pratumpong, P.; Pechyen, C. Preparation and foaming behavior of poly (lactic acid)/poly (butylene succinate)/cellulose fiber composite for hot cups packaging application. Food Packag. Shelf Life 2021, 27, 100608. [Google Scholar] [CrossRef]
- Yang, W.; Weng, Y.; Puglia, D.; Qi, G.; Dong, W.; Kenny, J.M.; Ma, P. Poly(lactic acid)/lignin films with enhanced toughness and anti-oxidation performance for active food packaging. Int. J. Biol. Macromol. 2020, 144, 102–110. [Google Scholar] [CrossRef]
- Min, T.; Sun, X.; Yuan, Z.; Zhou, L.; Jiao, X.; Zha, J.; Zhu, Z.; Wen, Y. Novel antimicrobial packaging film based on porous poly(lactic acid) nanofiber and polymeric coating for humidity-controlled release of thyme essential oil. Lwt 2021, 135, 110034. [Google Scholar] [CrossRef]
- Rizal, S.; Olaiya, F.G.; Saharudin, N.I.; Abdullah, C.K.; Olaiya, N.G.; Mohamad Haafiz, M.K.; Yahya, E.B.; Sabaruddin, F.A.; Ikramullah; Abdul Khalil, H.P.S. Isolation of textile waste cellulose nanofibrillated fibre reinforced in polylactic acid-chitin biodegradable composite for green packaging application. Polymers 2021, 13, 325. [Google Scholar] [CrossRef] [PubMed]
- Pirsa, S.; Asadi, S. Innovative smart and biodegradable packaging for margarine based on a nano composite polylactic acid/lycopene film. Food Addit. Contam. Part A Chem. Anal. Control. Expo. Risk Assess. 2021, 38, 856–869. [Google Scholar] [CrossRef]
- Lu, R.; Sameen, D.E.; Qin, W.; Wu, D.; Dai, J.; Li, S.; Liu, Y. Development of polylactic acid films with selenium microparticles and its application for food packaging. Coatings 2020, 10, 280. [Google Scholar] [CrossRef] [Green Version]
- Aragón-Gutierrez, A.; Arrieta, M.P.; López-González, M.; Fernández-García, M.; López, D. Hybrid biocomposites based on poly(Lactic acid) and silica aerogel for food packaging applications. Materials 2020, 13, 4910. [Google Scholar] [CrossRef]
- Srisa, A.; Harnkarnsujarit, N. Antifungal films from trans-cinnamaldehyde incorporated poly(lactic acid) and poly(butylene adipate-co-terephthalate) for bread packaging. Food Chem. 2020, 333, 127537. [Google Scholar] [CrossRef]
- Nikvarz, N.; Khayati, G.R.; Sharafi, S. Preparation of UV absorbent films using polylactic acid and grape syrup for food packaging application. Mater. Lett. 2020, 276, 128187. [Google Scholar] [CrossRef]
- Nerantzaki, M.; Kehagias, N.; Francone, A.; Fernández, A.; Sotomayor Torres, C.M.; Papi, R.; Choli-Papadopoulou, T.; Bikiaris, D.N. Design of a Multifunctional Nanoengineered PLLA Surface by Maximizing the Synergies between Biochemical and Surface Design Bactericidal Effects. ACS Omega 2018, 3, 1509–1521. [Google Scholar] [CrossRef]
- Psochia, E.; Papadopoulos, L.; Gkiliopoulos, D.J.; Francone, A.; Grigora, M.-E.; Tzetzis, D.; de Castro, J.V.; Neves, N.M.; Triantafyllidis, K.S.; Torres, C.M.S.; et al. Bottom-Up Development of Nanoimprinted PLLA Composite Films with Enhanced Antibacterial Properties for Smart Packaging Applications. Macromol 2021, 1, 5. [Google Scholar] [CrossRef]
- Delgado-Aguilar, M.; Puig, R.; Sazdovski, I.; Fullana-i-Palmer, P. Polylactic acid/polycaprolactone blends: On the path to circular economy, substituting single-use commodity plastic products. Materials 2020, 13, 2655. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhu, B.; Wang, Y.; Liu, C.; Shen, C. Effect of different sterilization methods on the properties of commercial biodegradable polyesters for single-use, disposable medical devices. Mater. Sci. Eng. C 2019, 105, 110041. [Google Scholar] [CrossRef] [PubMed]
- Avinc, O.; Khoddami, A. Overview of poly(lactic acid) (PLA) fibre. Fibre Chem. 2009, 41, 391–401. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, M.; Ju, Z.; Tam, P.Y.; Hua, T.; Younas, M.W.; Kamrul, H.; Hu, H. Poly(lactic acid) fibers, yarns and fabrics: Manufacturing, properties and applications. Text. Res. J. 2020. [Google Scholar] [CrossRef]
- Jabbar, A.; Tausif, M.; Tahir, H.R.; Basit, A.; Bhatti, M.R.A.; Abbas, G. Polylactic acid/lyocell fibre as an eco-friendly alternative to polyethylene terephthalate/cotton fibre blended yarns and knitted fabrics. J. Text. Inst. 2020, 111, 129–138. [Google Scholar] [CrossRef]
- Sharma, S.; Majumdar, A.; Butola, B.S. International Journal of Biological Macromolecules Tailoring the biodegradability of polylactic acid (PLA) based fi lms and ramie- PLA green composites by using selective additives. Int. J. Biol. Macromol. 2021, 181, 1092–1103. [Google Scholar] [CrossRef]
- Maqsood, N.; Rimašauskas, M. Characterization of carbon fiber reinforced PLA composites manufactured by fused deposition modeling. Compos. Part C Open Access 2021, 4, 100112. [Google Scholar] [CrossRef]
- Pukánszky, B. Rheology of PLA/Regenerated Cellulose Nanocomposites Prepared by the Pickering Emulsion Process : Network Formation and. Mater. Des. 2021, 109774. [Google Scholar] [CrossRef]
- Battegazzore, D.; Abt, T.; Maspoch, M.L.; Frache, A. Multilayer cotton fabric bio-composites based on PLA and PHB copolymer for industrial load carrying applications. Compos. Part B Eng. 2019, 163, 761–768. [Google Scholar] [CrossRef]
- Kabirian, F.; Ditkowski, B.; Zamanian, A.; Heying, R.; Mozafari, M. An innovative approach towards 3D-printed scaffolds for the next generation of tissue-engineered vascular grafts. Mater. Today Proc. 2018, 5, 15586–15594. [Google Scholar] [CrossRef]
- Adesina, O.T.; Jamiru, T.; Sadiku, E.R.; Ogunbiyi, O.F.; Adegbola, T.A. Water absorption and thermal degradation behavior of graphene reinforced poly(lactic) acid nanocomposite. IOP Conf. Ser. Mater. Sci. Eng. 2019, 627. [Google Scholar] [CrossRef]
- Werchefani, M.; Lacoste, C.; Belguith, H.; Gargouri, A.; Bradai, C. Effect of chemical and enzymatic treatments of alfa fibers on polylactic acid bio-composites properties. J. Compos. Mater. 2020, 54, 4959–4967. [Google Scholar] [CrossRef]
- He, H.; Yang, P.; Duan, Z.; Wang, Z.; Liu, Y. Reinforcing effect of hybrid nano-coating on mechanical properties of basalt fiber / poly (lactic acid) environmental composites. Compos. Sci. Technol. 2020, 199, 108372. [Google Scholar] [CrossRef]
- Wang, G.; Zhang, D.; Wan, G.; Li, B.; Zhao, G. Glass fiber reinforced PLA composite with enhanced mechanical properties, thermal behavior, and foaming ability. Polymer (Guildf) 2019, 181. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef] [PubMed]
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. 2 Tilman. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef]
- Knoch, S.; Pelletier, F.; Larose, M.; Chouinard, G.; Dumont, M.J.; Tavares, J.R. Surface modification of PLA nets intended for agricultural applications. Colloids Surf. A Physicochem. Eng. Asp. 2020, 598, 124787. [Google Scholar] [CrossRef]
- Mukherjee, A.; Knoch, S.; Chouinard, G.; Tavares, J.R.; Dumont, M.J. Use of bio-based polymers in agricultural exclusion nets: A perspective. Biosyst. Eng. 2019, 180, 121–145. [Google Scholar] [CrossRef]
- Castellano, S.; Scarascia Mugnozza, G.; Russo, G.; Briassoulis, D.; Mistriotis, A.; Hemming, S.; Waaijenberg, D. Plastic Nets in Agriculture: A General Review of Types and Applications. Appl. Eng. Agric. 2008, 24, 799–808. [Google Scholar] [CrossRef]
- Miros-Kudra, P.; Gzyra-Jagieła, K.; Kudra, M. Physicochemical assessment of the biodegradability of agricultural nonwovens made of PLA. Fibres Text. East. Eur. 2021, 29, 26–34. [Google Scholar] [CrossRef]
- Zhang, M.; Jia, H.; Weng, Y.; Li, C. Biodegradable PLA/PBAT mulch on microbial community structure in different soils. Int. Biodeterior. Biodegrad. 2019, 145, 104817. [Google Scholar] [CrossRef]
- Ünkel, A.N.K.; Se, B.; Chlegel, K.A.S.; Se, B. Polymers, Biodegradable; Wiley: Hoboken, NJ, USA, 2016. [Google Scholar]
- Hayes, D.G.; Wadsworth, L.C.; Sintim, H.Y.; Flury, M.; English, M.; Schaeffer, S.; Saxton, A.M. Effect of diverse weathering conditions on the physicochemical properties of biodegradable plastic mulches. Polym. Test. 2017, 62, 454–467. [Google Scholar] [CrossRef]
- Rocha, D.B.; Souza de Carvalho, J.; de Oliveira, S.A.; dos Santos Rosa, D. A new approach for flexible PBAT/PLA/CaCO3 films into agriculture. J. Appl. Polym. Sci. 2018, 135, 1–9. [Google Scholar] [CrossRef]
- Yu, Y.; Xu, P.; Jia, S.; Pan, H.; Zhang, H.; Wang, D.; Dong, L. Exploring polylactide/poly(butylene adipate-co-terephthalate)/rare earth complexes biodegradable light conversion agricultural films. Int. J. Biol. Macromol. 2019, 127, 210–221. [Google Scholar] [CrossRef]
- Välimäki, M.K.; Sokka, L.I.; Peltola, H.B.; Ihme, S.S.; Rokkonen, T.M.J.; Kurkela, T.J.; Ollila, J.T.; Korhonen, A.T.; Hast, J.T. Printed and hybrid integrated electronics using bio-based and recycled materials—increasing sustainability with greener materials and technologies. Int. J. Adv. Manuf. Technol. 2020, 111, 325–339. [Google Scholar] [CrossRef]
- Bio-Polylactic Acid (PLA) Market—Growth, Trends, COVID-19; Globe Newswire: New York, NY, USA, 2021.
- Mattana, G.; Briand, D.; Marette, A.; Vásquez Quintero, A.; De Rooij, N.F. Polylactic acid as a biodegradable material for all-solution-processed organic electronic devices. Org. Electron. 2015, 17, 77–86. [Google Scholar] [CrossRef]
- Luoma, E.; Välimäki, M.; Rokkonen, T.; Sääskilahti, H.; Ollila, J.; Rekilä, J.; Immonen, K. Oriented and annealed poly(lactic acid) films and their performance in flexible printed and hybrid electronics. J. Plast. Film Sheeting 2021. [Google Scholar] [CrossRef]
- Abu Hassan, N.A.; Ahmad, S.; Chen, R.S.; Shahdan, D. Cells analyses, mechanical and thermal stability of extruded polylactic acid/kenaf bio-composite foams. Constr. Build. Mater. 2020, 240, 117884. [Google Scholar] [CrossRef]
- Barkhad, M.S.; Abu-Jdayil, B.; Iqbal, M.Z.; Mourad, A.H.I. Thermal insulation using biodegradable poly(lactic acid)/date pit composites. Constr. Build. Mater. 2020, 261, 120533. [Google Scholar] [CrossRef]
- Patil, A.Y.; Banapurmath, N.R.; Shivangi, U.S. Feasibility study of epoxy coated Poly Lactic Acid as a sustainable replacement for river sand. J. Clean. Prod. 2020, 267, 121750. [Google Scholar] [CrossRef]
- Dassanayaka, D.; Hedigalla, D.; Gunasekera, U. Biodegradable composite for temporary partitioning materials. In Proceedings of the 2020 Moratuwa Engineering Research Conference (MERCon), Moratuwa, Sri Lanka, 28–30 July 2020; pp. 453–458. [Google Scholar] [CrossRef]
- Savioli Lopes, M.; Jardini, A.L.; Maciel Filho, R. Poly (lactic acid) production for tissue engineering applications. Procedia Eng. 2012, 42, 1402–1413. [Google Scholar] [CrossRef] [Green Version]
- Kareem, M.M.; Hodgkinson, T.; Sanchez, M.S.; Dalby, M.J. Hybrid core—Shell scaffolds for bone tissue engineering. Biomed. Mater. 2019, 14, 025008. [Google Scholar] [CrossRef]
- da Silva, T.N.; Gonçalves, R.P.; Rocha, C.L.; Archanjo, B.S.; Barboza, C.A.G.; Pierre, M.B.R.; Reynaud, F.; de Souza Picciani, P.H. Controlling burst effect with PLA/PVA coaxial electrospun scaffolds loaded with BMP-2 for bone guided regeneration. Mater. Sci. Eng. C 2019, 97, 602–612. [Google Scholar] [CrossRef]
- Jaidev, L.R.; Chatterjee, K. Surface functionalization of 3D printed polymer scaffolds to augment stem cell response. Mater. Des. 2019, 161, 44–54. [Google Scholar] [CrossRef]
- Heinrich, M.A.; Liu, W.; Jimenez, A.; Yang, J.; Akpek, A.; Liu, X.; Pi, Q.; Mu, X.; Hu, N.; Schiffelers, R.M.; et al. 3D Bioprinting: From Benches to Translational Applications. Small 2019, 15, 1–47. [Google Scholar] [CrossRef]
- Coolen, A.L.; Lacroix, C.; Mercier-Gouy, P.; Delaune, E.; Monge, C.; Exposito, J.Y.; Verrier, B. Poly(lactic acid) nanoparticles and cell-penetrating peptide potentiate mRNA-based vaccine expression in dendritic cells triggering their activation. Biomaterials 2019, 195, 23–37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, B.; Wang, L.; Song, P.; Pei, X.; Sun, H.; Wu, L.; Zhou, C.; Wang, K.; Fan, Y.; Zhang, X. 3D printed bone tissue regenerative PLA/HA scaffolds with comprehensive performance optimizations. Mater. Des. 2021, 201, 109490. [Google Scholar] [CrossRef]
- Liu, Z.; Ge, Y.; Zhang, L.; Wang, Y.; Guo, C.; Feng, K.; Yang, S.; Zhai, Z.; Chi, Y.; Zhao, J.; et al. The effect of induced membranes combined with enhanced bone marrow and 3D PLA-HA on repairing long bone defects in vivo. J. Tissue Eng. Regen. Med. 2020, 14, 1403–1414. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, B.N.; Aprile, P.; Mendonça, R.H.; Kelly, D.J.; da Silva Moreira Thiré, R.M. Evaluation of bone marrow stem cell response to PLA scaffolds manufactured by 3D printing and coated with polydopamine and type I collagen. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 37–49. [Google Scholar] [CrossRef] [Green Version]
- Yao, Q.; Liu, Y.; Sun, H. Heparin–dopamine functionalized graphene foam for sustained release of bone morphogenetic protein-2. J. Tissue Eng. Regen. Med. 2018, 12, 1519–1529. [Google Scholar] [CrossRef] [PubMed]
- Mao, D.; Li, Q.; Bai, N.; Dong, H.; Li, D. Porous stable poly(lactic acid)/ethyl cellulose/hydroxyapatite composite scaffolds prepared by a combined method for bone regeneration. Carbohydr. Polym. 2018, 180, 104–111. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic-co-glycolic) Acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef]
- Xie, R.; Hu, J.; Hoffmann, O.; Zhang, Y.; Ng, F.; Qin, T.; Guo, X. Self-fitting shape memory polymer foam inducing bone regeneration: A rabbit femoral defect study. Biochim. Biophys. Acta Gen. Subj. 2018, 1862, 936–945. [Google Scholar] [CrossRef]
- Kankala, R.K.; Zhao, J.; Liu, C.G.; Song, X.J.; Yang, D.Y.; Zhu, K.; Wang, S.B.; Zhang, Y.S.; Chen, A.Z. Highly porous microcarriers for minimally invasive in situ skeletal muscle cell delivery. Small 2019, 15, 1–15. [Google Scholar] [CrossRef]
- Yin, S.; Zhang, W.; Zhang, Z.; Jiang, X. Recent advances in scaffold design and material for vascularized tissue-engineered bone regeneration. Adv. Healthc. Mater. 2019, 8, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Sartore, L.; Inverardi, N.; Pandini, S.; Bignotti, F.; Chiellini, F. PLA/PCL-based foams as scaffolds for tissue engineering applications. Mater. Today Proc. 2019, 7, 410–417. [Google Scholar] [CrossRef]
- Elsayed, H.; Rebesan, P.; Crovace, M.C.; Zanotto, E.D.; Colombo, P.; Bernardo, E. Biosilicate® scaffolds produced by 3D-printing and direct foaming using preceramic polymers. J. Am. Ceram. Soc. 2019, 102, 1010–1020. [Google Scholar] [CrossRef] [Green Version]
- Mao, D.; Li, Q.; Li, D.; Chen, Y.; Chen, X.; Xu, X. Fabrication of 3D porous poly(lactic acid)-based composite scaffolds with tunable biodegradation for bone tissue engineering. Mater. Des. 2018, 142, 1–10. [Google Scholar] [CrossRef]
- Pavia, F.C.; Conoscenti, G.; Greco, S.; La Carrubba, V.; Ghersi, G.; Brucato, V. Preparation, characterization and in vitro test of composites poly-lactic acid/hydroxyapatite scaffolds for bone tissue engineering. Int. J. Biol. Macromol. 2018, 119, 945–953. [Google Scholar] [CrossRef] [PubMed]
- Kaliaraj, R.; Gandhi, S.; Sundaramurthi, D.; Sethuraman, S.; Krishnan, U.M. A biomimetic mesoporous silica–polymer composite scaffold for bone tissue engineering. J. Porous Mater. 2018, 25, 397–406. [Google Scholar] [CrossRef]
- Diaz-Gomez, L.; García-González, C.A.; Wang, J.; Yang, F.; Aznar-Cervantes, S.; Cenis, J.L.; Reyes, R.; Delgado, A.; Évora, C.; Concheiro, A.; et al. Biodegradable PCL/fibroin/hydroxyapatite porous scaffolds prepared by supercritical foaming for bone regeneration. Int. J. Pharm. 2017, 527, 115–125. [Google Scholar] [CrossRef]
- Novotna, L.; Kucera, L.; Hampl, A.; Drdlik, D.; Cihlar, J. Biphasic calcium phosphate scaffolds with controlled pore size distribution prepared by in-situ foaming. Mater. Sci. Eng. C 2019, 95, 363–370. [Google Scholar] [CrossRef]
- Luo, Y.; Humayun, A.; Mills, D.K. Surface modification of 3d printed pla/halloysite composite scaffolds with antibacterial and osteogenic capabilities. Appl. Sci. 2020, 10, 3971. [Google Scholar] [CrossRef]
- Zou, L.; Zhang, Y.; Liu, X.; Chen, J.; Zhang, Q. Biomimetic mineralization on natural and synthetic polymers to prepare hybrid scaffolds for bone tissue engineering. Colloids Surf. B Biointerfaces 2019, 178, 222–229. [Google Scholar] [CrossRef]
- Romanova, O.A.; Tenchurin, T.H.; Demina, T.S.; Sytina, E.V.; Shepelev, A.D.; Rudyak, S.G.; Klein, O.I.; Krasheninnikov, S.V.; Safronova, E.I.; Kamyshinsky, R.A.; et al. Non-woven bilayered biodegradable chitosan-gelatin-polylactide scaffold for bioengineering of tracheal epithelium. Cell Prolif. 2019, 52, e12598. [Google Scholar] [CrossRef]
- Rogina, A.; Antunović, M.; Milovac, D. Biomimetic design of bone substitutes based on cuttlefish bone-derived hydroxyapatite and biodegradable polymers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 197–204. [Google Scholar] [CrossRef] [Green Version]
- Sharif, F.; Tabassum, S.; Mustafa, W.; Asif, A.; Zarif, F.; Tariq, M.; Siddiqui, S.A.; Gilani, M.A.; Ur Rehman, I.; MacNeil, S. Bioresorbable antibacterial PCL-PLA-nHA composite membranes for oral and maxillofacial defects. Polym. Compos. 2019, 40, 1564–1575. [Google Scholar] [CrossRef]
- Sun, S.; Li, Q.; Zhao, N.; Jiang, J.; Zhang, K.; Hou, J.; Wang, X.; Liu, G. Preparation of highly interconnected porous poly(ε-caprolactone)/poly(lactic acid) scaffolds via supercritical foaming. Polym. Adv. Technol. 2018, 29, 3065–3074. [Google Scholar] [CrossRef]
- Beslikas, T.; Gigis, I.; Goulios, V.; Christoforides, J.; Papageorgiou, G.Z.; Bikiaris, D.N. Crystallization study and comparative in vitro-in vivo hydrolysis of PLA reinforcement ligament. Int. J. Mol. Sci. 2011, 12, 6597–6618. [Google Scholar] [CrossRef] [Green Version]
- Papageorgiou, G.Z.; Theodore, B.; Johngigis, J.C.; Bikiaris, D.N. Crystallization and Enzymatic Hydrolysis of PLA Grade for Orthopedics. Adv. Polym. Technol. 2010, 29, 280–299. [Google Scholar] [CrossRef]
- Liu, S.; Qin, S.; He, M.; Zhou, D.; Qin, Q.; Wang, H. Current applications of poly(lactic acid) composites in tissue engineering and drug delivery. Compos. Part B Eng. 2020, 199, 108238. [Google Scholar] [CrossRef]
- Li, X.; Zhang, W.; Lin, W.; Qiu, H.; Qi, Y.; Ma, X.; Qi, H.; He, Y.; Zhang, H.; Qian, J.; et al. Long-Term Efficacy of Biodegradable Metal-Polymer Composite Stents after the First and the Second Implantations into Porcine Coronary Arteries. ACS Appl. Mater. Interfaces 2020, 12, 15703–15715. [Google Scholar] [CrossRef] [PubMed]
- Zamproni, L.N.; Grinet, M.A.V.M.; Mundim, M.T.V.V.; Reis, M.B.C.; Galindo, L.T.; Marciano, F.R.; Lobo, A.O.; Porcionatto, M. Rotary jet-spun porous microfibers as scaffolds for stem cells delivery to central nervous system injury. Nanomed. Nanotechnol. Biol. Med. 2019, 15, 98–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Chen, B.; Zhao, H.; Yu, P.; Fu, D.; Wen, J.; Peng, X. Processing and characterization of supercritical CO2 batch foamed poly(lactic acid)/poly(ethylene glycol) scaffold for tissue engineering application. J. Appl. Polym. Sci. 2013, 130, 3066–3073. [Google Scholar] [CrossRef]
- Gomaa, S.F.; Madkour, T.M.; Moghannem, S.; El-Sherbiny, I.M. New polylactic acid/ cellulose acetate-based antimicrobial interactive single dose nanofibrous wound dressing mats. Int. J. Biol. Macromol. 2017, 105, 1148–1160. [Google Scholar] [CrossRef]
- Lalloz, A.; Bolzinger, M.A.; Briançon, S.; Faivre, J.; Rabanel, J.M.; Garcia Ac, A.; Hildgen, P.; Banquy, X. Subtle and unexpected role of PEG in tuning the penetration mechanisms of PLA-based nano-formulations into intact and impaired skin. Int. J. Pharm. 2019, 563, 79–90. [Google Scholar] [CrossRef]
- Zhang, S.; Ye, J.; Sun, Y.; Kang, J.; Liu, J.; Wang, Y.; Li, Y.; Zhang, L.; Ning, G. Electrospun fibrous mat based on silver (I) metal-organic frameworks-polylactic acid for bacterial killing and antibiotic-free wound dressing. Chem. Eng. J. 2020, 390, 124523. [Google Scholar] [CrossRef]
- Cui, S.; Sun, X.; Li, K.; Gou, D.; Zhou, Y.; Hu, J.; Liu, Y. Polylactide nanofibers delivering doxycycline for chronic wound treatment. Mater. Sci. Eng. C 2019, 104. [Google Scholar] [CrossRef]
- Fang, Y.; Zhu, X.; Wang, N.; Zhang, X.; Yang, D.; Nie, J.; Ma, G. Biodegradable core-shell electrospun nanofibers based on PLA and γ-PGA for wound healing. Eur. Polym. J. 2019, 116, 30–37. [Google Scholar] [CrossRef]
- Kumar, R.; Jha, D.; Panda, A.K. Poly-Lactide/Poly-Lactide-co-Glycolide-Based Delivery System for Bioactive Compounds against Microbes; Springer: Cham, Switzerland, 2019; pp. 75–98. [Google Scholar] [CrossRef]
- Qin, S.Y.; Zhang, A.Q.; Zhang, X.Z. Recent advances in targeted tumor chemotherapy based on smart nanomedicines. Small 2018, 14, 1–24. [Google Scholar] [CrossRef]
- Jelonek, K.; Zajdel, A.; Wilczok, A.; Latocha, M.; Musiał-Kulik, M.; Foryś, A.; Kasperczyk, J. Dual-targeted biodegradable micelles for anticancer drug delivery. Mater. Lett. 2019, 241, 187–189. [Google Scholar] [CrossRef]
- Li, W.; Fan, X.; Wang, X.; Shang, X.; Wang, Q.; Lin, J.; Hu, Z.; Li, Z. Stereocomplexed micelle formation through enantiomeric PLA-based Y-shaped copolymer for targeted drug delivery. Mater. Sci. Eng. C 2018, 91, 688–695. [Google Scholar] [CrossRef]
- Oz, U.C.; Bolat, Z.B.; Ozkose, U.U.; Gulyuz, S.; Kucukturkmen, B.; Khalily, M.P.; Ozcubukcu, S.; Yilmaz, O.; Telci, D.; Esendagli, G.; et al. A robust optimization approach for the breast cancer targeted design of PEtOx-b-PLA polymersomes. Mater. Sci. Eng. C 2021, 123, 111929. [Google Scholar] [CrossRef]
- Dixit, S.; Sahu, R.; Verma, R.; Duncan, S.; Giambartolomei, G.H.; Singh, S.R.; Dennis, V.A. Caveolin-mediated endocytosis of the Chlamydia M278 outer membrane peptide encapsulated in poly(lactic acid)-Poly(ethylene glycol) nanoparticles by mouse primary dendritic cells enhances specific immune effectors mediated by MHC class II and CD4+ T cells. Biomaterials 2018, 159, 130–145. [Google Scholar] [CrossRef]
- Qu, M.; Xiao, W.; Tian, J.; Wang, S.; Li, H.; Liu, X.; Yang, X.; Li, B.; Liao, X. Fabrication of superparamagnetic nanofibrous poly (l-lactic acid)/gamma-Fe2O3 microspheres for cell carriers. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 511–520. [Google Scholar] [CrossRef] [PubMed]
- Varga, N.; Hornok, V.; Janovák, L.; Dékány, I.; Csapó, E. The effect of synthesis conditions and tunable hydrophilicity on the drug encapsulation capability of PLA and PLGA nanoparticles. Colloids Surf. B Biointerfaces 2019, 176, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Wang, X.Q.; Zhang, Q.; Zhu, J.Y.; Li, Y.; Xie, C.F.; Li, X.T.; Wu, J.S.; Geng, S.S.; Zhong, C.Y.; et al. (−)-Epigallocatechin-3-gallate inhibits colorectal cancer stem cells by suppressing Wnt/β-catenin pathway. Nutrients 2017, 9, 572. [Google Scholar] [CrossRef] [Green Version]
- Ito, F.; Honnami, H.; Kawakami, H.; Kanamura, K.; Makino, K. Preparation and properties of PLGA microspheres containing hydrophilic drugs by the SPG (shirasu porous glass) membrane emulsification technique. Colloids Surf. B Biointerfaces 2008, 67, 20–25. [Google Scholar] [CrossRef]
- Sun, S.; Du, X.; Fu, M.; Khan, A.R.; Ji, J.; Liu, W.; Zhai, G. Galactosamine-modified PEG-PLA/TPGS micelles for the oral delivery of curcumin. Int. J. Pharm. 2021, 595, 120227. [Google Scholar] [CrossRef]
- Yan, T.; Ma, Z.; Liu, J.; Yin, N.; Lei, S.; Zhang, X.; Li, X.; Zhang, Y.; Kong, J. Thermoresponsive GenisteinNLC-dexamethasone-moxifloxacin multi drug delivery system in lens capsule bag to prevent complications after cataract surgery. Sci. Rep. 2021, 11, 1–14. [Google Scholar] [CrossRef]
- Liu, R.; Chen, Y.; Liu, L.; Gong, Y.; Wang, M.; Li, S.; Chen, C.; Yu, B. Long-term delivery of rhIGF-1 from biodegradable poly(lactic acid)/hydroxyapatite@Eudragit double-layer microspheres for prevention of bone loss and articular degeneration in C57BL/6 mice. J. Mater. Chem. B 2018, 6, 3085–3095. [Google Scholar] [CrossRef]
- Zykova, Y.; Kudryavtseva, V.; Gai, M.; Kozelskaya, A.; Frueh, J.; Sukhorukov, G.; Tverdokhlebov, S. Free-standing microchamber arrays as a biodegradable drug depot system for implant coatings. Eur. Polym. J. 2019, 114, 72–80. [Google Scholar] [CrossRef]
- Dwivedi, A.; Mazumder, A.; Nasongkla, N. Layer-by-layer nanocoating of antibacterial niosome on orthopedic implant. Int. J. Pharm. 2018, 547, 235–243. [Google Scholar] [CrossRef]
- Nanaki, S.; Viziridou, A.; Zamboulis, A.; Kostoglou, M.; Papageorgiou, G.Z.; Bikiaris, D.N. New Biodegradable Poly (l-lactide)-Block- for Long-Acting Injectables of Naltrexone Drug. Polymers 2020, 12, 852. [Google Scholar] [CrossRef] [Green Version]
- Christodoulou, E.; Nerantzaki, M.; Nanaki, S.; Barmpalexis, P.; Anastasiou, A.D.; Bikiaris, D.N. Paclitaxel magnetic core—Shell nanoparticles based on poly(lactic acid) semitelechelic novel block copolymers for combined hyperthermia and chemotherapy treatment of cancer. Pharmaceutics 2019, 11, 213. [Google Scholar] [CrossRef] [Green Version]
- Nanaki, S.; Siafaka, P.I.; Zachariadou, D.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.S.; Kostoglou, M.; Nikolakaki, E.; Bikiaris, D.N. PLGA/SBA-15 mesoporous silica composite microparticles loaded with paclitaxel for local chemotherapy. Eur. J. Pharm. Sci. 2017, 99, 32–44. [Google Scholar] [CrossRef]
- Nanaki, S.; Tseklima, M.; Terzopoulou, Z.; Nerantzaki, M.; Giliopoulos, D.J.; Triantafyllidis, K.; Kostoglou, M.; Bikiaris, D.N. Use of mesoporous cellular foam (MCF) in preparation of polymeric microspheres for long acting injectable release formulations of paliperidone antipsychotic drug. Eur. J. Pharm. Biopharm. 2017, 117, 77–90. [Google Scholar] [CrossRef]
- Nanaki, S.; Barmpalexis, P.; Iatrou, A.; Christodoulou, E.; Kostoglou, M.; Bikiaris, D.N. Risperidone controlled release microspheres based on poly(lactic acid)-poly(propylene adipate) novel polymer blends appropriate for long acting injectable formulations. Pharmaceutics 2018, 10, 130. [Google Scholar] [CrossRef] [Green Version]
- Karavelidis, V.; Giliopoulos, D.; Karavas, E.; Bikiaris, D. Nanoencapsulation of a water soluble drug in biocompatible polyesters. Effect of polyesters melting point and glass transition temperature on drug release behavior. Eur. J. Pharm. Sci. 2010, 41, 636–643. [Google Scholar] [CrossRef]
- Ye, K.; Liu, D.; Kuang, H.; Cai, J.; Chen, W.; Sun, B.; Xia, L.; Fang, B.; Morsi, Y.; Mo, X. Three-dimensional electrospun nanofibrous scaffolds displaying bone morphogenetic protein-2-derived peptides for the promotion of osteogenic differentiation of stem cells and bone regeneration. J. Colloid Interface Sci. 2019, 534, 625–636. [Google Scholar] [CrossRef]
- Martin, V.; Ribeiro, I.A.; Alves, M.M.; Gonçalves, L.; Claudio, R.A.; Grenho, L.; Fernandes, M.H.; Gomes, P.; Santos, C.F.; Bettencourt, A.F. Engineering a multifunctional 3D-printed PLA-collagen-minocycline-nanoHydroxyapatite scaffold with combined antimicrobial and osteogenic effects for bone regeneration. Mater. Sci. Eng. C 2019, 101, 15–26. [Google Scholar] [CrossRef]
- Ramesh, N.; Moratti, S.C.; Dias, G.J. Hydroxyapatite–polymer biocomposites for bone regeneration: A review of current trends. J. Biomed. Mater. Res. Part B Appl. Biomater. 2018, 106, 2046–2057. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Radmansouri, M.; Habibi Jouybari, M.; Ghasemi, M.V.; Mohammadi, A.; Irani, M.; Jazi, F.S. Incorporation of magnetic NaX zeolite/DOX into the PLA/chitosan nanofibers for sustained release of doxorubicin against carcinoma cells death in vitro. Int. J. Biol. Macromol. 2019, 121, 398–406. [Google Scholar] [CrossRef] [PubMed]
- Karamanlioglu, M.; Preziosi, R.; Robson, G.D. The Compostable Plastic Poly(lactic) Acid Causes a Temporal Shift in Fungal Communities in Maturing Compost. Compost Sci. Util. 2017, 25, 211–219. [Google Scholar] [CrossRef]
- Cosate de Andrade, M.F.; Souza, P.M.S.; Cavalett, O.; Morales, A.R. Life cycle assessment of poly(lactic acid) (PLA): Comparison between chemical recycling, mechanical recycling and composting. J. Polym. Environ. 2016, 24, 372–384. [Google Scholar] [CrossRef]
- Newport, E.; Chen, Z. Biodegradable Plastics: Breaking Down the Facts—Production, Composition and Environmental Impact; Green Peace East Asia: Hong Kong, China, 2020; 54p. [Google Scholar]
- Kjeldsen, A.; Price, M.; Lilley, C.; Guzniczak, E.; Archer, I. A Review of Standards for Biodegradable Plastics; Industrial Biotechnology Innovation Centtre IbioIC: Glasgow, UK, 2019; 33p. [Google Scholar]
- Is PLA Filament Actually Biodegradable? 3Dnatives: New York, NY, USA, 2019.
- Is PLA Biodegradable What Is PLA and What Is It Made of? 3Dnatives: New York, NY, USA, 2019.
- Qi, X.; Ren, Y.; Wang, X. New advances in the biodegradation of Poly(lactic) acid. Int. Biodeterior. Biodegrad. 2017, 117, 215–223. [Google Scholar] [CrossRef]
- Emadian, S.M.; Onay, T.T.; Demirel, B. Biodegradation of bioplastics in natural environments. Waste Manag. 2017, 59, 526–536. [Google Scholar] [CrossRef]
- Bellego, C.L.; Oxand, I.S.A.; Montigny-Sur-Loing, D.C. Simplified modeling of calcium leaching of concrete in various environments. Mater. Struct. 2002, 3, 632–640. [Google Scholar]
- Satti, S.M.; Shah, A.A.; Marsh, T.L.; Auras, R. Biodegradation of Poly(lactic acid) in Soil Microcosms at Ambient Temperature: Evaluation of Natural Attenuation, Bio-augmentation and Bio-stimulation. J. Polym. Environ. 2018, 26, 3848–3857. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Degradation behaviour of poly(lactic acid) films and fibres in soil under Mediterranean field conditions and laboratory simulations testing. Ind. Crops Prod. 2011, 33, 648–658. [Google Scholar] [CrossRef]
- Rudnik, E.; Briassoulis, D. Comparative Biodegradation in Soil Behaviour of two Biodegradable Polymers Based on Renewable Resources. J. Polym. Environ. 2011, 19, 18–39. [Google Scholar] [CrossRef]
- Chamas, A.; Moon, H.; Zheng, J.; Qiu, Y.; Tabassum, T.; Jang, J.H.; Abu-Omar, M.; Scott, S.L.; Suh, S. Degradation Rates of Plastics in the Environment. ACS Sustain. Chem. Eng. 2020, 8, 3494–3511. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Wang, B.; Yang, G.; Gauthier, M. Poly(lactic acid)—Based biomaterials: Synthesis, modification and applications. Biomed. Sci. Eng. Technol. 2012, 11, 247–282. [Google Scholar]
- Sanusi, O.M.; Benelfellah, A.; Bikiaris, D.N.; Aït Hocine, N. Effect of rigid nanoparticles and preparation techniques on the performances of poly(lactic acid) nanocomposites: A review. Polym. Adv. Technol. 2021, 32, 444–460. [Google Scholar] [CrossRef]
- Masutani, K.; Kimura, Y. Present situation and future perspectives of poly(lactic acid). Adv. Polym. Sci. 2018, 279, 1–25. [Google Scholar] [CrossRef]
- John, R.P.; Anisha, G.S.; Nairc, N.R.; Nampoothiri, K.M. Poly lactic acid: An environmentally friendly biodegradable polymer. Biodegrad. Mater. Prod. Prop. Appl. 2011, 79–90. [Google Scholar]












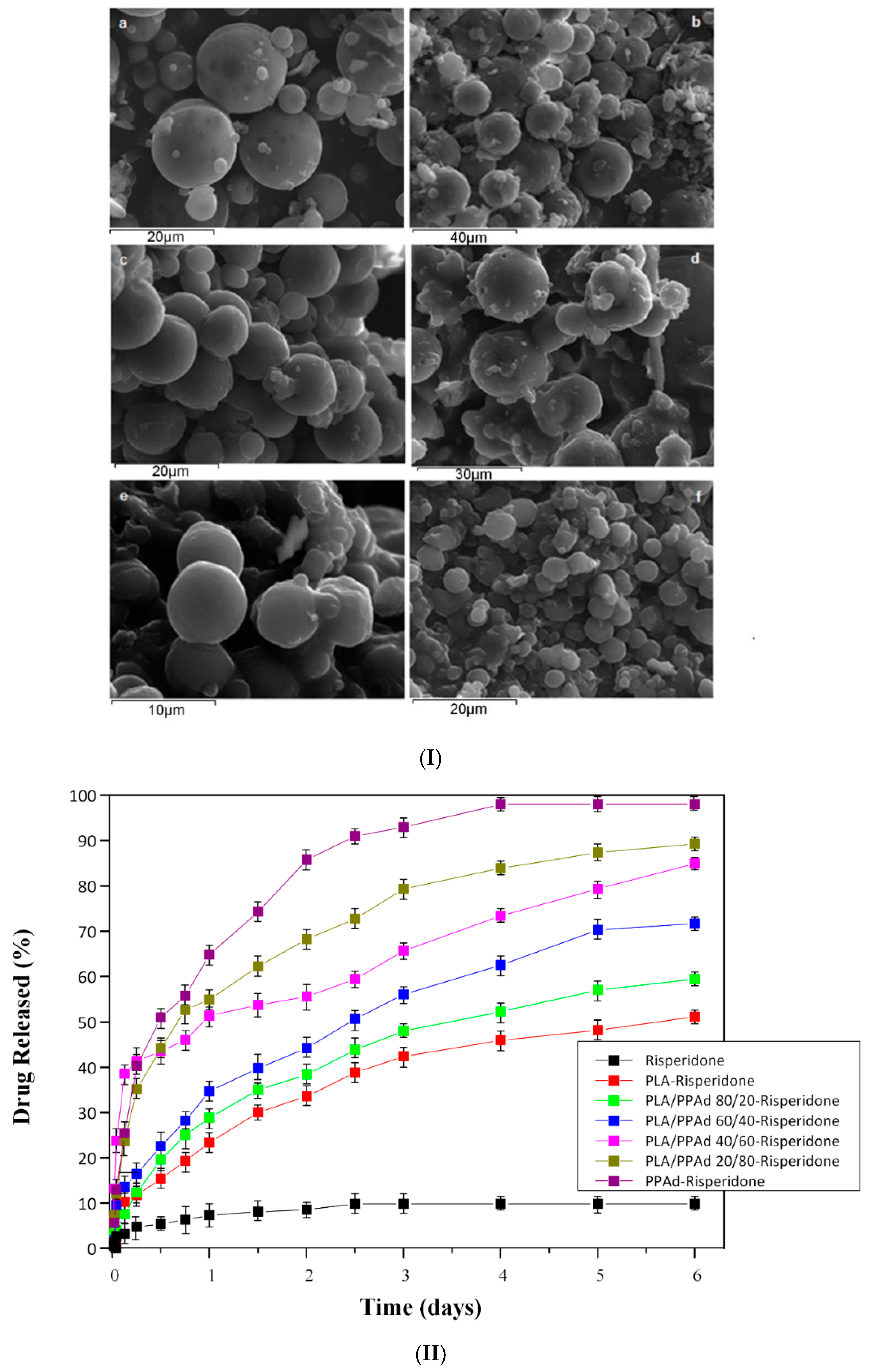
| Run | Catalyst | Catalyst/OLLA (wt%) | Temperature (°C) | Time (h) | Pressure (torr) | MW (g/mol) | Yield (%) |
|---|---|---|---|---|---|---|---|
| 1 | GeO2 | 0.8 | 180 | 20 | 10 | 28,000 | 73 |
| 2 | Sb2O3 | 0.1 | 200 | 30 | 20 | 20,000 | 25 |
| 3 | ZnO | 0.1 | 200 | 30 | 20 | 36,000 | 35 |
| 4 | Fe2O3 | 0.1 | 200 | 8 | 1 | 20,000 | 23 |
| 5 | Al2O3 | 8.5 | 200 | 30 | 20 | 27,000 | 42 |
| 6 | SiO2 | 0.8 | 180 | 20 | 10 | 11,000 | 58 |
| 7 | TiO2 | 0.8 | 180 | 20 | 10 | 11,000 | 64 |
| 8 | SnO | 0.2 | 180 | 20 | 10 | 50,000 | 36 |
| 9 | SnCl2·2H2O | 0.4 | 180 | 20 | 10 | 41,000 | 43 |
| 10 | TSA | 0.34 | 180 | 10 | 10 | 17,000 | 70 |
| PLA/Composite | Improvement in Properties Achieved | Application | Refs. |
|---|---|---|---|
| PLA/ZnO membranes | Mechanical properties, UV, visiblelight barrier performances. | Food packaging | [125] |
| PLA/Chitosan/Basil olive oil films | Tensile, barrier, and antioxidant properties. | Active food packaging | [126] |
| PLA/poly(butylene succinate)/cellulose fiber composite | Viscosity, Thermal properties, crystallinity, flexural modulus. | Hot cups or lids application | [127] |
| PLA/lignin films | Toughness, antioxidant performance, water vapor barrier properties, antioxidant, UV resistance behavior | Active food packaging | [128] |
| PLA/poly(vinyl alcohol)/poly(ethylene glycol) blends/thyme essential oil | Hydrophilicity, antibacterial properties | Antimicrobial packaging | [129] |
| PLA/chitin/cellulose nanofiber | Mechanical, thermal, and wettability properties | Green packaging | [130] |
| PLA/lycopene nanocomposite film | Mechanical properties, oxidative and color stability of margarine | Margarine packaging | [131] |
| PLA/selenium microparticles films | Water resistance, ultraviolet resistance, antibacterial and oxidation resistance | Food packaging | [132] |
| PLA/silica aerogel composite | Crystallinity, stretchability, elongation at break, high transparency | Food packaging | [133] |
| PLA/halloysite nanotubes bionanocomposite | Thermal properties, mechanical properties (tensile strength, yield strength). | Food packaging | [122] |
| PLA/poly(butylene adipate-co-terephthalate) (PBAT) blends with incorporated trans-cinnamaldehyde | Antimicrobial properties, antifungal properties. | Bread packaging | [134] |
| PLA/cinnamaldehyde inclusions films | Antibacterial properties, tensile strength, water and oxygen resistance, life expectancy. | Fruit packaging | [123] |
| PLA/grape syrup films | UV absorption and light stability | Food packaging | [135] |
| PLA/Composite | Improvement in Properties Achieved | Refs. |
|---|---|---|
| PLA/Ramie | PLA brittleness reduced Acceleration of PLA’s biodegradation rate Reduction of carbon footprint | [143] |
| PLA/CCF (continuous carbon fiber) | High tensile strength | [144] |
| PLA/Regenerated cellulose | Improved rheological characteristics | [145] |
| PLA/cotton fabric | Improved flexural properties | [146] |
| PLA Fiber Reinforcement | Improvements in the Properties Achieved | Application | References |
|---|---|---|---|
| Alfa fibers | Tensile strength | Ecological and economical components | [149] |
| Basalt fibers | Mechanical properties | Excellent strength and fracture toughness components | [150] |
| Glass fibers | Mechanical properties, Thermal behavior, Foaming ability | Light weight structural components | [151] |
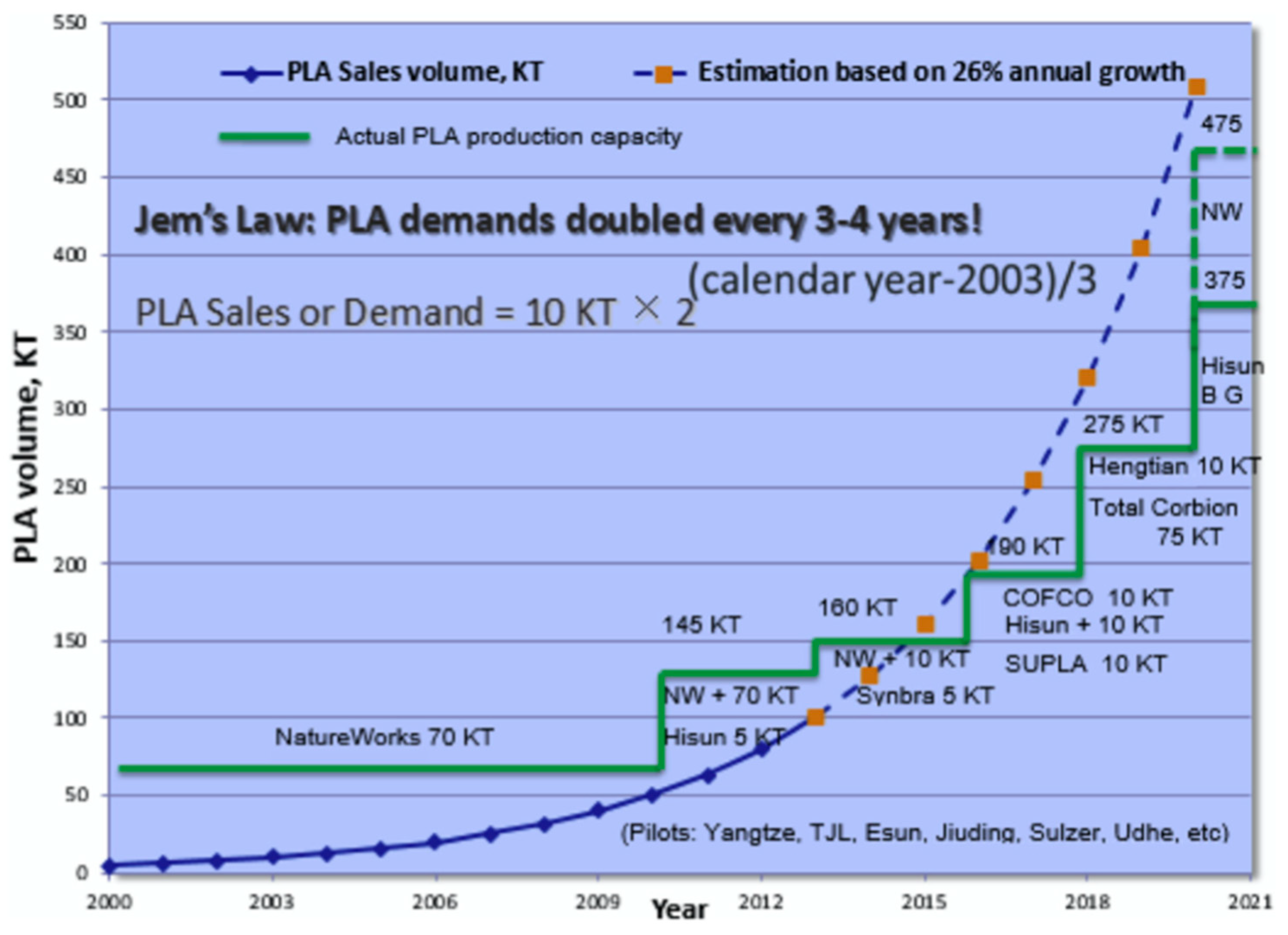
| Key PLA Projects in 2018–2020 | Location of Operation | KT |
|---|---|---|
| NatureWorks | USA | 150 |
| Total Corbion PLA JV | Thailand | 75 |
| Hisun | China | 45 |
| BBCA & Galactic | China | 40 |
| COFCO | China | 10 |
| Hengtian | China | 10 |
| SuPLA | China | 10 |
| TongJieLiang | China | 10 |
| Synbra | Netherlands | 5 |
| TianRen | China | 3 |
| Futerro (under Galactic) | Belgium | 1 |
| Jiangxi KeYuan | China | 1 |
| Sulzer | Switzerland | <1 |
| Pyramid (under Udhe) | Germany | <1 |
| Other announced projects (e.g., XinNing, HongDa, TongBang, YouCheng) | China | N/A |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Balla, E.; Daniilidis, V.; Karlioti, G.; Kalamas, T.; Stefanidou, M.; Bikiaris, N.D.; Vlachopoulos, A.; Koumentakou, I.; Bikiaris, D.N. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers 2021, 13, 1822. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111822
Balla E, Daniilidis V, Karlioti G, Kalamas T, Stefanidou M, Bikiaris ND, Vlachopoulos A, Koumentakou I, Bikiaris DN. Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications. Polymers. 2021; 13(11):1822. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111822
Chicago/Turabian StyleBalla, Evangelia, Vasileios Daniilidis, Georgia Karlioti, Theocharis Kalamas, Myrika Stefanidou, Nikolaos D. Bikiaris, Antonios Vlachopoulos, Ioanna Koumentakou, and Dimitrios N. Bikiaris. 2021. "Poly(lactic Acid): A Versatile Biobased Polymer for the Future with Multifunctional Properties—From Monomer Synthesis, Polymerization Techniques and Molecular Weight Increase to PLA Applications" Polymers 13, no. 11: 1822. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111822