Biodegradable Polymeric Foams Based on Modified Castor Oil, Styrene, and Isobornyl Methacrylate
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Castor Oil Glycerides and Maleated Castor Oil Glycerides
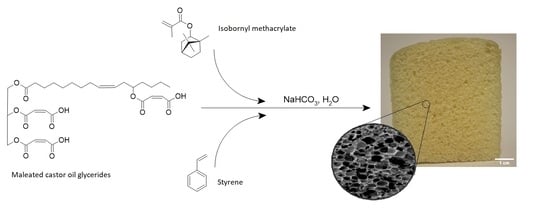
2.3. Preparation of Polymeric Foams
2.4. Characterization Techniques
2.5. Biodegradability of Polymeric Foams
2.6. Statistical Analysis
3. Results and Discussion
3.1. Synthesis of Castor Oil Glycerides and Maleated Castor Oil Glycerides
3.2. Preparation of Polymeric Foams
3.3. Polymeric Foam Cell Morphology
3.4. Compressive Properties
3.5. Biodegradability
4. Summary and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Islam, M.R.; Beg, M.D.H.; Jamari, S.S. Development of vegetable-oil-based polymers. J. Appl. Polym. Sci. 2014, 131. [Google Scholar] [CrossRef] [Green Version]
- Kundu, P.P.; Das, R. Development of biobased polymers and their composites from vegetable oils. J. Biodegrad. Biobased Polym. Environ. Biomed. Appl. 2016, 287–320. [Google Scholar] [CrossRef]
- Miao, S.; Wang, P.; Su, Z.; Zhang, S. Vegetable-oil-based polymers as future polymeric biomaterials. Acta Biomater. 2014, 10, 1692–1704. [Google Scholar] [CrossRef]
- Mustapha, R.; Rahmat, A.R.; Majid, R.A.; Mustapha, S.N.H. Mustapha, Vegetable oil-based epoxy resins and their composites with bio-based hardener: A short review. Polym. Technol. Mater. 2019, 58, 1311–1326. [Google Scholar]
- Mustapha, S.N.H.; Rahmat, A.R.; Arsad, A. Bio-based thermoset nanocomposite derived from vegetable oil: A short review. Rev. Chem. Eng. 2014, 30, 167–182. [Google Scholar] [CrossRef]
- Petrović, Z.S. Polyurethanes from vegetable oils. Polym. Rev. 2008, 48, 109–155. [Google Scholar] [CrossRef]
- Tan, S.G.; Chow, W.S. Biobased epoxidized vegetable oils and its greener epoxy blends: A review. Polym. Technol. Eng. 2010, 49, 1581–1590. [Google Scholar] [CrossRef]
- Galià, M.; de Espinosa, L.M.; Ronda, J.C.; Lligadas, G.; Cádiz, V. Vegetable oil-based thermosetting polymers. Eur. J. Lipid Sci. Technol. 2010, 112, 87–96. [Google Scholar] [CrossRef]
- Sharma, V.; Kundu, P. Addition polymers from natural oils—A review. Prog. Polym. Sci. 2006, 31, 983–1008. [Google Scholar] [CrossRef]
- Lu, P.; Guo, M.; Yang, Y.; Wu, M. Nanocellulose stabilized pickering emulsion templating for thermosetting AESO nanocomposite foams. Polymers 2018, 10, 1111. [Google Scholar] [CrossRef] [Green Version]
- Sousa, A.F.; Ferreira, S.; Lopez, A.; Borges, I.; Pinto, R.; Silvestre, A.J.; Freire, C.S. Freire, Thermosetting AESO-bacterial cellulose nanocomposite foams with tailored mechanical properties obtained by Pickering emulsion templating. Polymer 2017, 118, 127–134. [Google Scholar] [CrossRef]
- Bonnaillie, L.M. Bio-Based Polymeric Foam from Soybean Oil and Carbon Dioxide. Ph.D. Thesis, Department of Chemical Engineering, University of Delaware, Newark, DE, USA, 2007. [Google Scholar]
- Bonnaillie, L.M.; Wool, R.P. Thermosetting foam with a high bio-based content from acrylated epoxidized soybean oil and carbon dioxide. J. Appl. Polym. Sci. 2007, 105, 1042–1052. [Google Scholar] [CrossRef]
- Wu, S.P.; Qiu, J.F.; Rong, M.Z.; Zhang, M.Q.; Zhang, L.Y. Plant oil-based biofoam composites with balanced performance. Polym. Int. 2009, 58, 403–411. [Google Scholar] [CrossRef]
- Rong, M.Z.; Zhang, M.Q.; Wu, S.P.; Wang, H.J.; Czigány, T. Ecomaterials-Foam Plastics Synthesized from Plant Oil-Based Resins, Materials Science Forum; Trans Tech Publications Ltd.: Zurich, Switzerland, 2007; pp. 2311–2316. [Google Scholar]
- Lee, K.-Y.; Wong, L.L.C.; Blaker, J.; Hodgkinson, J.M.; Bismarck, A. Bismarck, Bio-based macroporous polymer nanocomposites made by mechanical frothing of acrylated epoxidised soybean oil. Green Chem. 2011, 13, 3117–3123. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.F.; Zhang, M.Q.; Rong, M.Z.; Wu, S.P.; Karger-Kocsis, J. Rigid bio-foam plastics with intrinsic flame retardancy derived from soybean oil. J. Mater. Chem. A 2013, 1, 2533–2542. [Google Scholar] [CrossRef] [Green Version]
- Campanella, A.; La Scala, J.J.; Wool, R.P. The use of acrylated fatty acid methyl esters as styrene replacements in triglyceride-based thermosetting polymers. Polym. Eng. Sci. 2009, 49, 2384–2392. [Google Scholar] [CrossRef]
- Cousinet, S.; Ghadban, A.; Fleury, E.; Lortie, F.; Pascault, J.-P.; Portinha, D. Portinha, Toward replacement of styrene by bio-based methacrylates in unsaturated polyester resins. Eur. Polym. J. 2015, 67, 539–550. [Google Scholar] [CrossRef]
- Yadav, S.K.; Schmalbach, K.M.; Kinaci, E.; Stanzione, J.F.; Palmese, G.R. Palmese, Recent advances in plant-based vinyl ester resins and reactive diluents. Eur. Polym. J. 2018, 98, 199–215. [Google Scholar] [CrossRef]
- Yu, A.Z.; Serum, E.M.; Renner, A.C.; Sahouani, J.M.; Sibi, M.P.; Webster, D.C. Webster, Renewable Reactive Diluents as Practical Styrene Replacements in Biobased Vinyl Ester Thermosets. ACS Sustain. Chem. Eng. 2018, 6, 12586–12592. [Google Scholar] [CrossRef]
- Wang, H.J.; Rong, M.Z.; Zhang, M.Q.; Hu, J.; Chen, H.W.; Czigány, T. Biodegradable foam plastics based on castor oil. Biomacromolecules 2007, 9, 615–623. [Google Scholar] [CrossRef]
- Wang, H.J.; Zhang, C.Y.; Rong, M.Z.; Zhang, M.Q.; Czigány, T. Interfacial effects in short sisal fiber/maleated castor oil foam composites. Compos. Interfaces 2008, 15, 95–110. [Google Scholar] [CrossRef]
- Can, E.; Wool, R.P.; Küsefoğlu, S. Soybean and castor oil based monomers: Synthesis and copolymerization with styrene. J. Appl. Polym. Sci. 2006, 102, 2433–2447. [Google Scholar] [CrossRef]
- Can, E.; Wool, R.; Küsefoğlu, S. Soybean-and castor-oil-based thermosetting polymers: Mechanical properties. J. Appl. Polym. Sci. 2006, 102, 1497–1504. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Perez, W.A.; Rios, L.A. Synthesis of maleated-castor oil glycerides from biodiesel-derived crude glycerol. Ind. Crop. Prod. 2013, 49, 299–303. [Google Scholar] [CrossRef]
- Echeverri, D.A.; Rios, L.A.; Rivas, B.L. Synthesis and copolymerization of thermosetting resins obtained from vegetable oils and biodiesel-derived crude glycerol. Eur. Polym. J. 2015, 67, 428–438. [Google Scholar] [CrossRef]
- Campanella, A.; La Scala, J.J.; Wool, R.P. Fatty acid-based comonomers as styrene replacements in soybean and castor oil-based thermosetting polymers. J. Appl. Polym. Sci. 2010, 119, 1000–1010. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Acid and Base Number by Color-Indicator Titration; ASTM D974-14e2; ASTM International: West Conshohocken, PA, USA, 2014. [Google Scholar]
- ASTM. Standard Test Method for Determination of the Saponification Value of Fats and Oils; ASTM D5558-95; ASTM International: West Conshohocken, PA, USA, 2017. [Google Scholar]
- Huber, A.T.; Gibson, L.J. Anisotropy of foams. J. Mater. Sci. 1988, 23, 3031–3040. [Google Scholar] [CrossRef]
- Shutov, F.A. Foamed Polymers. Cellular Structure and Properties. In Industrial Developments; Springer: Berlin/Heidelberg, Germany, 1983; pp. 155–218. [Google Scholar]
- Aleksandrov, A.Y.; Borodin, M.Y.; Pavlov, V. Constructions with Plastic Foam Fillers; Mashinostroenie: Moscow, Russia, 1972. (In Russian) [Google Scholar]
- ASTM. Standard Test Method for Compressive Properties of Rigid Cellular Plastics; ASTM D1621-16; ASTM International: West Conshohocken, PA, USA, 2016. [Google Scholar]
- Li, Q.M.; Magkiriadis, I.; Harrigan, J.J. Compressive strain at the onset of densification of cellular solids. J. Cell. Plast. 2006, 42, 371–392. [Google Scholar] [CrossRef]
- Tan, P.; Reid, S.; Harrigan, J.; Zou, Z.; Li, S. Dynamic compressive strength properties of aluminium foams. Part I—Experimental data and observations. J. Mech. Phys. Solids 2005, 53, 2174–2205. [Google Scholar] [CrossRef]
- ASTM. Standard Test Method for Determining Aerobic Biodegradation of Plastic Materials in Soil; ASTM D5988-18; ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- Obi, B. Polymeric Foams Structure-Property-Performance: A Design Guide; William Andrew: Oxford, UK, 2017. [Google Scholar]
- Shukla, S.; Agnihotri, S.; Pradeep, S.A.; Pilla, S. Delayed addition foaming of bio-epoxy blends: Balancing performance requirements and sustainability. ACS Sustain. Chem. Eng. 2018, 6, 17051–17058. [Google Scholar] [CrossRef]
- Brown, N.F.; Pradeep, S.A.; Agnihotri, S.; Pilla, S. The power of processing: Creating high strength foams from epoxidized pine oil. ACS Sustain. Chem. Eng. 2017, 5, 8641–8647. [Google Scholar] [CrossRef]
- Agnihotri, S.; Shukla, S.; Pradeep, S.A.; Pilla, S. Biobased thermosetting cellular blends: Exploiting the ecological advantage of epoxidized soybean oil in structural foams. Polymer 2019, 177, 111–119. [Google Scholar] [CrossRef]
- Badía, A.; Movellan, J.; Barandiaran, M.J.; Leiza, J.R. High Biobased Content Latexes for Development of Sustainable Pressure Sensitive Adhesives. Ind. Eng. Chem. Res. 2018, 57, 14509–14516. [Google Scholar] [CrossRef]
- Slone, R.V. Methacrylic Ester Polymers. Encycl. Polym. Sci. Technol. 2002. [Google Scholar] [CrossRef]
- Altuna, F.I.; Ruseckaite, R.A.; Stefani, P.M. Biobased thermosetting epoxy foams: Mechanical and thermal characterization. ACS Sustain. Chem. Eng. 2015, 3, 1406–1411. [Google Scholar] [CrossRef]
- Mazzon, E.; Habas-Ulloa, A.; Habas, J.-P. Lightweight rigid foams from highly reactive epoxy resins derived from vegetable oil for automotive applications. Eur. Polym. J. 2015, 68, 546–557. [Google Scholar] [CrossRef]
- Huang, X.; Yang, X.; Liu, H.; Shang, S.; Cai, Z.; Wu, K. Bio-based thermosetting epoxy foams from epoxidized soybean oil and rosin with enhanced properties. Ind. Crop. Prod. 2019, 139, 111540. [Google Scholar] [CrossRef]
- Lau, T.H.; Wong, L.L.; Lee, K.-Y.; Bismarck, A. Tailored for simplicity: Creating high porosity, high performance bio-based macroporous polymers from foam templates. Green Chem. 2013, 16, 1931–1940. [Google Scholar] [CrossRef] [Green Version]
- Gibson, L.J.; Ashby, M.F. Cellular Solids: Structure and Properties, 2nd ed.; Cambridge University Press: Cambridge, UK, 1999. [Google Scholar]
- Chen, Y.; Das, R.; Battley, M. Modelling of closed-cell foams incorporating cell size and cell wall thickness variations. In Proceedings of the 11th World Congress on Computational Mechanics, Barcelona, Spain, 20–25 July 2014; pp. 20–25. [Google Scholar]
- Nie, Z.; Lin, Y.; Tong, Q. Modeling structures of open cell foams. Comput. Mater. Sci. 2017, 131, 160–169. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, R.; Rodrigue, D. Cell morphology analysis of high density polymer foams. Polym. Test. 2005, 24, 1027–1035. [Google Scholar] [CrossRef]
- Lautensack, C. Fitting three-dimensional Laguerre tessellations to foam structures. J. Appl. Stat. 2008, 35, 985–995. [Google Scholar] [CrossRef]
- Wong, J.C.; Tervoort, E.; Busato, S.; Gonzenbach, U.T.; Studart, A.R.; Ermanni, P.; Gauckler, L.J. Macroporous polymers from particle-stabilized foams. J. Mater. Chem. 2009, 19, 5129–5133. [Google Scholar] [CrossRef]
- Yin, D.; Xiang, A.; Li, Y.; Qi, H.; Tian, H.; Fan, G. Effect of Plasticizer on the Morphology and Foaming Properties of Poly(vinyl alcohol) Foams by Supercritical CO2 Foaming Agents. J. Polym. Environ. 2019, 27, 2878–2885. [Google Scholar] [CrossRef]
- Oliveira-Salmazo, L.; Lopez-Gil, A.; Silva-Bellucci, F.; Job, A.E.; Rodriguez-Perez, M.A. Natural rubber foams with anisotropic cellular structures: Mechanical properties and modeling. Ind. Crop. Prod. 2016, 80, 26–35. [Google Scholar] [CrossRef] [Green Version]
- Tu, Z.; Shim, V.; Lim, C. Plastic deformation modes in rigid polyurethane foam under static loading. Int. J. Solids Struct. 2001, 38, 9267–9279. [Google Scholar] [CrossRef]
- Thirumal, M.; Khastgir, D.; Singha, N.K.; Manjunath, B.S.; Naik, Y.P. Effect of foam density on the properties of water blown rigid polyurethane foam. J. Appl. Polym. Sci. 2008, 108, 1810–1817. [Google Scholar] [CrossRef]
- Członka, S.; Sienkiewicz, N.; Kairytė, A.; Vaitkus, S. Colored polyurethane foams with enhanced mechanical and thermal properties. Polym. Test. 2019, 78, 105986. [Google Scholar] [CrossRef]
- Marvi-Mashhadi, M.; Lopes, C.; Llorca, J. Effect of anisotropy on the mechanical properties of polyurethane foams: An experimental and numerical study. Mech. Mater. 2018, 124, 143–154. [Google Scholar] [CrossRef]
- Bernardo, V.; Laguna-Gutierrez, E.; Lopez-Gil, A.; Rodriguez-Perez, M.A. Highly anisotropic crosslinked HDPE foams with a controlled anisotropy ratio: Production and characterization of the cellular structure and mechanical properties. Mater. Des. 2017, 114, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Kidd, T.; Zhuang, S.; Ravichandran, G. In situ mechanical characterization during deformation of PVC polymeric foams using ultrasonics and digital image correlation. Mech. Mater. 2012, 55, 82–88. [Google Scholar] [CrossRef]
- Lim, G.; Altstädt, V.; Ramsteiner, F. Understanding the compressive behavior of linear and cross-linked poly (vinyl chloride) foams. J. Cell. Plast. 2009, 45, 419–439. [Google Scholar] [CrossRef]
- Lee, J.-H.; Kim, S.-K.; Park, S.; Park, K.H.; Lee, J.-M. Unified constitutive model with consideration for effects of porosity and its application to polyurethane foam. Compos. Part B Eng. 2018, 138, 87–100. [Google Scholar] [CrossRef]
- Sanders, W.; Gibson, L. Mechanics of hollow sphere foams. Mater. Sci. Eng. A 2003, 347, 70–85. [Google Scholar] [CrossRef]
- Stengard, R. Properties of Rigid Urethane Foams; EI duPont de Nemours and Co.: Wilmington, NC, USA, 1963. [Google Scholar]
- Stefani, P.; Barchi, A.T.; Sabugal, J.; Vazquez, A. Characterization of epoxy foams. J. Appl. Polym. Sci. 2003, 90, 2992–2996. [Google Scholar] [CrossRef]
- Goods, S.; Neuschwanger, C.; Whinnery, L.; Nix, W. Mechanical properties of a particle-strengthened polyurethane foam. J. Appl. Polym. Sci. 1999, 74, 2724–2736. [Google Scholar] [CrossRef]
- Song, C.; Wang, P.; Makse, H.A. A phase diagram for jammed matter. Nature 2008, 453, 629. [Google Scholar] [CrossRef] [PubMed]
- Carriço, C.S.; Fraga, T.; Pasa, V.M. Production and characterization of polyurethane foams from a simple mixture of castor oil, crude glycerol and untreated lignin as bio-based polyols. Eur. Polym. J. 2016, 85, 53–61. [Google Scholar] [CrossRef]
- Tondi, G.; Link, M.; Kolbitsch, C.; Gavino, J.; Luckeneder, P.; Petutschnigg, A.; Herchl, R.; van Doorslaer, C. Lignin-based foams: Production process and characterization. BioResources 2016, 11, 2972–2986. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Rahimidehgolan, F.; Altenhof, W. Anisotropic compressive behavior of rigid PVC foam at strain rates up to 200 s−1. Polym. Test. 2020, 91, 106836. [Google Scholar] [CrossRef]
- Sousa, A.F.; Matos, M.; Pinto, R.J.; Freire, C.S.; Silvestre, A.J. One-pot synthesis of biofoams from castor oil and cellulose microfibers for energy absorption impact materials. Cellulose 2014, 21, 1723–1733. [Google Scholar] [CrossRef]
- del Rosso, S.; Iannucci, L. On the compressive response of polymeric cellular materials. Materials 2020, 13, 457. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Linul, E.; Şerban, D.-A.; Voiconi, T.; Marşavina, L.; Sadowski, T. Energy–absorption and efficiency diagrams of rigid PUR foams. Key Eng. Mater. 2014, 601, 246–249. [Google Scholar] [CrossRef]
- Qiu, D.; He, Y.; Yu, Z. Investigation on compression mechanical properties of rigid polyurethane foam treated under random vibration condition: An experimental and numerical simulation study. Materials 2019, 12, 3385. [Google Scholar] [CrossRef] [Green Version]
- Da Silva, V.R.; Mosiewicki, M.A.; Yoshida, M.I.; da Silva, M.C.; Stefani, P.M.; Marcovich, N.E. Polyurethane foams based on modified tung oil and reinforced with rice husk ash II: Mechanical characterization. Polym. Test. 2013, 32, 665–672. [Google Scholar] [CrossRef]
- Koohbor, B.; Kidane, A. Design optimization of continuously and discretely graded foam materials for efficient energy absorption. Mater. Des. 2016, 102, 151–161. [Google Scholar] [CrossRef]
- Saha, M.; Mahfuz, H.; Chakravarty, U.; Uddin, M.; Kabir, M.; Jeelani, S. Effect of density, microstructure, and strain rate on compression behavior of polymeric foams. Mater. Sci. Eng. A 2005, 406, 328–336. [Google Scholar] [CrossRef]
- Woodard, L.N.; Grunlan, M.A. Hydrolytic degradation and erosion of polyester biomaterials. ACS Macro Lett. 2018, 7, 976–982. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.J.; Edelman, P.G. An overview of biodegradable polymers and biodegradation of polymers. Degrad. Polym. 1995, 18–28. [Google Scholar] [CrossRef]
- Pischedda, A.; Tosin, M.; Degli-Innocenti, F. Biodegradation of plastics in soil: The effect of temperature. Polym. Degrad. Stab. 2019, 170, 109017. [Google Scholar] [CrossRef]
- Liszkowska, J.; Borowicz, M.; Paciorek-Sadowska, J.; Isbrandt, M.; Czupryński, B.; Moraczewski, K. Assessment of photodegradation and biodegradation of RPU/PIR foams modified by natural compounds of plant origin. Polymers 2019, 12, 33. [Google Scholar] [CrossRef] [Green Version]
- Álvarez-Barragán, J.; Domínguez-Malfavón, L.; Vargas-Suárez, M.; González-Hernández, R.; Aguilar-Osorio, G.; Loza-Tavera, H. Biodegradative activities of selected environmental fungi on a polyester polyurethane varnish and polyether polyurethane foams. Appl. Environ. Microbiol. 2016, 82, 5225. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Styrene (wt%) | IBOMA (wt%) | NaHCO3 (phr) | H2O (phr) | Foaming Method | |
|---|---|---|---|---|---|
| PF1a | 30 | 0 | 1.75 | 4 | 1 |
| PF1b | 30 | 0 | 3.0 | 4 | 1 |
| PF1c | 30 | 0 | 1.5 | 4 | 2a |
| PF1d | 30 | 0 | 2.0 | 4 | 1 |
| PF2a | 15 | 15 | 2.25 | 4 | 2b |
| PF2b | 15 | 15 | 1.5 | 4 | 2b |
| PF3a | 0 | 50 | 2.25 | 4 | 1 |
| PF3b | 0 | 50 | 0.1 | 0.5 | 1, 2a, 2b |
| ρ* (g/cm3) | e | Dav‖ (µm) | Dav˫ (µm) | R | δav‖ (µm) | δav˫ (µm) | |
|---|---|---|---|---|---|---|---|
| PF1a | 0.189 ± 0.003 | 0.83 | 612 ± 24 | 417 ± 16 | 1.47 | 60 | 41 |
| PF1b | 0.125 ± 0.006 | 0.89 | 709 ± 34 | 551 ± 29 | 1.29 | 43 | 33 |
| PF1c | 0.241 ± 0.007 | 0.78 | 409 ± 21 | 274 ± 12 | 1.49 | 54 | 36 |
| PF1d | 0.168 ± 0.004 | 0.85 | 222 ± 8 | 192 ± 7 | 1.16 | 19 | 16 |
| PF2a | 0.168 ± 0.004 | 0.85 | 410 ± 17 | 384 ± 19 | 1.07 | 35 | 33 |
| PF2b | 0.309 ± 0.008 | 0.73 | 246 ± 11 | 195 ± 7 | 1.26 | 42 | 33 |
| Ec (MPa) | σprop (MPa) | σcol (MPa) | σstr (MPa) | σplat (MPa) | Recovery (%) | |
|---|---|---|---|---|---|---|
| PF1a ParFR | 26.85 ± 1.07 | 0.70 ± 0.069 | 1.15 ± 0.066 | 1.11 ± 0.021 | 1.79 ± 0.172 | 54 ± 1.6 |
| PF1a PerFR | 15.69 ± 0.65 | 0.38 ± 0.023 | 0.57 ± 0.014 | 0.64 ± 0.033 | 0.80 ± 0.033 | 65 ± 1.7 |
| PF1b ParFR | 8.37 ± 0.46 | 0.25 ± 0.023 | 0.40 ± 0.015 | 0.39 ± 0.019 | 1.25 ± 0.33 | 60 ± 1.9 |
| PF1b PerFR | 3.51 ± 0.43 | 0.11 ± 0.0079 | 0.18 ± 0.019 | 0.19 ± 0.017 | 0.20 ± 0.017 | 86 ± 1.0 |
| PF1c | 23.31 ± 1.49 | 0.57 ± 0.011 | 1.00 ± 0.038 | 1.01 ± 0.044 | 1.05 ± 0.064 | 81 ± 1.0 |
| PF1d | 20.13 ± 1.73 | 0.57 ± 0.14 | 0.73 ± 0.12 | 0.79 ± 0.0761 | 1.01 ± 0.090 | 56 ± 1.0 |
| PF2a | 1.13 ± 0.107 | 0.048 ± 0.0033 | 0.072 ± 0.010 | 0.075 ± 0.0053 | 0.083 ± 0.0066 | 79 ± 1.0 |
| PF2b | 1.38 ± 0.055 | 0.057 ± 0.0039 | 0.085 ± 0.0030 | 0.088 ± 0.0031 | 0.092 ± 0.0031 | 71 ± 0.9 |
| Orientation | εOD (%) | WE at OD (%) | WOD (J/cm3) | Wtot (J/cm3) | |
|---|---|---|---|---|---|
| PF1a | ParFR | 38.0 ± 0.364 | 27.41 ± 0.16 | 40.22 ± 1.85 | 124.4 ± 7.93 |
| PerFR | 29.01 ± 0.63 | 17.77 ± 0.21 | 20.39 ± 0.96 | 120.02 ± 1.77 | |
| PF1b | ParFR | 41.9 ± 0.83 | 27.35 ± 1.76 | 18.36 ± 1.68 | 41.17 ± 5.43 |
| PerFR | 53.28 ± 0.78 | 33.29 ± 0.71 | 11.82 ± 0.90 | 14.61 ± 1.32 | |
| PF1c | ParFR | 49.16 ± 0.53 | 30.48 ± 0.63 | 56.02 ± 2.52 | 81.53 ± 4.27 |
| PF1d | ParFR | 31.86 ± 1.92 | 17.45 ± 0.34 | 29.69 ± 1.24 | 134.66 ± 1.98 |
| PF2a | ParFR | 48.97 ± 0.99 | 27.31 ± 0.43 | 4.53 ± 0.312 | 8.22 ± 0.28 |
| PF2b | ParFR | 44.69 ± 0.49 | 25.35 ± 0.64 | 4.18 ± 0.20 | 8.18 ± 0.58 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dicks, J.A.; Woolard, C. Biodegradable Polymeric Foams Based on Modified Castor Oil, Styrene, and Isobornyl Methacrylate. Polymers 2021, 13, 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111872
Dicks JA, Woolard C. Biodegradable Polymeric Foams Based on Modified Castor Oil, Styrene, and Isobornyl Methacrylate. Polymers. 2021; 13(11):1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111872
Chicago/Turabian StyleDicks, James Anthony, and Chris Woolard. 2021. "Biodegradable Polymeric Foams Based on Modified Castor Oil, Styrene, and Isobornyl Methacrylate" Polymers 13, no. 11: 1872. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111872