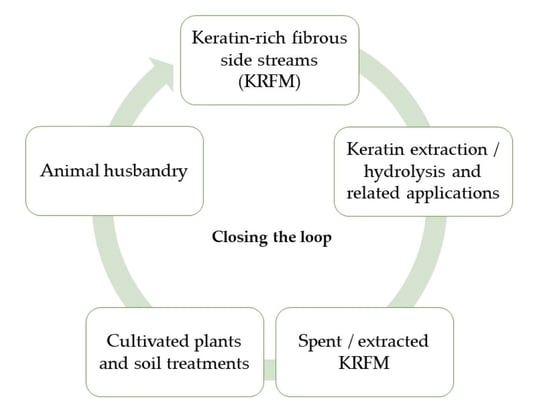
Closing the Loop with Keratin-Rich Fibrous Materials
Abstract
:1. Introduction
2. Composition and Structure of Keratin
2.1. α-Keratin
2.2. β-Keratin
2.3. γ-Keratin
2.4. Classification of Keratins
2.5. Properties and Functions
3. Methods for the Valorization of Keratins by Extraction
- Considering the effect on the native structure of keratin extraction methods can be grouped in protected solubilization (extracted keratin molecules are approximately intact) and unprotected solubilization (molecular structure of keratin is destroyed by degrading peptide bonds, disulfide bridges, and intermolecular hydrogen bonds resulting in a low molecular weight proteins and polypeptides mixture) [64,65];
- About the used extraction methods there are chemical (reduction, oxidation, hydrolysis, sulfitolysis, and the use of ionic liquid), physical (steam explosion, superheated water treatment, and microwave irradiation), and biological (microbial and enzymatic) methods [66];
- Regarding the effect on the environment, some green methods have been developed, such as microwave irradiation, supercritical water extraction, and steam explosion.
3.1. Chemical Methods for Keratin Extraction
3.1.1. Oxidative Extraction
3.1.2. Reductive Extraction
3.1.3. Sulfitolysis
3.1.4. Alkaline Extraction
3.1.5. Extraction with Ionic Liquids and Deep Eutectic Solvents
3.2. Enzymatic and Microbial Methods
3.3. Physical Methods for Keratin Extraction
3.3.1. Steam Explosion Extraction
3.3.2. Microwave Treatment
3.3.3. Superheated Water
4. Applications of Extracted Keratin
4.1. Cosmetic Applications
4.2. Biomedical Applications
4.3. Environmental Applications
4.4. Agricultural Applications
| Keratin Rich Side Stream | Manufacturing Process | Agricultural Application | Reference |
|---|---|---|---|
| Wool | Superheated water hydrolysis (170 °C, 60 min, solid to liquid ratio close to 1) | Nitrogen fertilizer | [159] |
| Wool | Alkaline hydrolysis (80 °C, 12% NaOH, 4 h, solid to liquid ratio 1:3) of the degreased wool | Nitrogen fertilizer | [15] |
| Feathers | Steam hydrolysis (135 °C, 90 min, solid to liquid ratio 2:1), followed by enzymatic hydrolysis (50 °C deg. 48–120 h, solid to liquid ratio 1:1) | Nitrogen fertilizer | [160] |
| Feathers | Coating produced from chicken feathers’ protein, acrylic acid, and N,N′-methylenebisacrylamide | Coating materials for controlled-release fertilizers; water retention/superadsorbent coating | [161] |
| Bovine hair | Keratin based superadsorbent coating produced from extracted keratin from waste bovine hair, acrylic acid, N,N-methylenebis acrylamide | Coating materials for controlled-release fertilizers; water retention/superadsorbent coating | [137] |
| Feathers | Solubilization of keratin in deep eutectic solvents (60 °C, 2 h, solid to liquid ratio 1:1), followed by dilution to water till 50% water and enzymatic hydrolysis with commercial protease (Alcalase, pH 8.2, 60 °C, 2 h). Resulted hydrolysate was coated into NPK fertilizer granules in a fluid bed granulator | Coating materials for controlled-release fertilizers | [162] |
| Bovine hair | Keratin extracted from bovine hair, included into a film-forming formulation, polyvinyl alcohol, polyacrylamide, N,N-methylenebis (acrylamide) and glycerol (GL) to prepare the low-cost degradable keratin-based sprayable mulch film | Sprayable mulch, with water holding capacity (380%) and high mineral nutrient content | [138] |
| Feathers | Supernatant produced by degradation of feathers by Chryseobacterium sp. RBT (accession number GU481093), grown in basalt salt medium and feathers, for 30 h, on rotary shaker (140 rpm) at 37 °C. | Plant biostimulant effects; quality improvement of banana produced by banana plant treated with feather hydrolysate | [163] |
| Feathers | Supernatant produced by degradation of feathers by Trichoderma asperellum T50 and T. atroviride grown in minimal medium and feathers, for 21 days, at 26 ± 2 °C and constant agitation—130 rpm | Plant biostimulant effects; activation of the proton pump, tomato seedling stimulation | [29] |
| Feathers | Supernatant produced by degradation of feathers by Bacillus aerius NSMk2 grown in a minimal media with 1.375%, pH 7.5, at 35 °C, for 3 days | Plant biostimulant effects; enhance mung bane growth and development | [164] |
4.5. Emerging Applications
5. Perspectives on Utilization of Extracted Keratin-Rich Fibrous Materials
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chaitanya Reddy, C.; Khilji, I.A.; Gupta, A.; Bhuyar, P.; Mahmood, S.; Saeed Al-Japairai, K.A.; Chua, G.K. Valorization of keratin waste biomass and its potential applications. J. Water Process Eng. 2021, 40, 101707. [Google Scholar] [CrossRef]
- Meyers, M.A.; Chen, P.-Y.; Lin, A.Y.-M.; Seki, Y. Biological materials: Structure and mechanical properties. Prog. Mater. Sci. 2008, 53, 1–206. [Google Scholar] [CrossRef] [Green Version]
- McKittrick, J.; Chen, P.Y.; Bodde, S.G.; Yang, W.; Novitskaya, E.E.; Meyers, M.A. The structure, functions, and mechanical properties of keratin. JOM 2012, 64, 449–468. [Google Scholar] [CrossRef]
- Tomlinson, D.J.; Mülling, C.H.; Fakler, T.M. Invited review: Formation of keratins in the bovine claw: Roles of hormones, minerals, and vitamins in functional claw integrity. J. Dairy Sci. 2004, 87, 797–809. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, A.; Rodrigues, R.M.; Machado, R.; Pereira, R.N.; Cavaco-Paulo, A.; Ribeiro, A. Ohmic heating as an innovative approach for the production of keratin films. Int. J. Biol. Macromol. 2020, 150, 671–680. [Google Scholar] [CrossRef] [Green Version]
- Herrmann, H.; Aebi, U. Intermediate filaments: Molecular structure, assembly mechanism, and integration into functionally distinct intracellular scaffolds. Annu. Rev. Biochem. 2004, 73, 749–789. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; McKittrick, J.; Meyers, M.A. Keratin: Structure, mechanical properties, occurrence in biological organisms, and efforts at bioinspiration. Prog. Mater. Sci. 2016, 76, 229–318. [Google Scholar] [CrossRef] [Green Version]
- Mulling, C. Three-dimensional appearance of bovine epidermal keratinocytes in different stages of differentiation revealed by cell maceration and scanning electron microscope investigation. Folia Morphol. 2000, 59, 239–246. [Google Scholar]
- Sharma, S.; Gupta, A. Sustainable management of keratin waste biomass: Applications and future perspectives. Braz. Arch. Biol. Technol. 2016, 59, e16150684. [Google Scholar] [CrossRef] [Green Version]
- Qiu, J.; Wilkens, C.; Barrett, K.; Meyer, A.S. Microbial enzymes catalyzing keratin degradation: Classification, structure, function. Biotechnol. Adv. 2020, 44, 107607. [Google Scholar] [CrossRef] [PubMed]
- Chao, S.J.; Chung, K.H.; Lai, Y.F.; Lai, Y.K.; Chang, S.H. Keratin particles generated from rapid hydrolysis of waste feathers with green des/koh: Efficient adsorption of fluoroquinolone antibiotic and its reuse. Int. J. Biol. Macromol. 2021, 173, 211–218. [Google Scholar] [CrossRef]
- Grigsby, W.J.; Scott, S.M.; Plowman-Holmes, M.I.; Middlewood, P.G.; Recabar, K. Combination and processing keratin with lignin as biocomposite materials for additive manufacturing technology. Acta Biomater. 2020, 104, 95–103. [Google Scholar] [CrossRef]
- Hussain, F.S.; Memon, N.; Khatri, Z.; Memon, S. Solid waste-derived biodegradable keratin sponges for removal of chromium: A circular approach for waste management in leather industry. Environ. Technol. Innov. 2020, 20, 101120. [Google Scholar] [CrossRef]
- Suarato, G.; Contardi, M.; Perotto, G.; Heredia-Guerrero, J.A.; Fiorentini, F.; Ceseracciu, L.; Pignatelli, C.; Debellis, D.; Bertorelli, R.; Athanassiou, A. From fabric to tissue: Recovered wool keratin/polyvinylpyrrolidone biocomposite fibers as artificial scaffold platform. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 116, 111151. [Google Scholar] [CrossRef]
- Gaidau, C.; Epure, D.-G.; Enascuta, C.E.; Carsote, C.; Sendrea, C.; Proietti, N.; Chen, W.; Gu, H. Wool keratin total solubilisation for recovery and reintegration-an ecological approach. J. Clean. Prod. 2019, 236, 117586. [Google Scholar] [CrossRef]
- Thankaswamy, S.R.; Sundaramoorthy, S.; Palanivel, S.; Ramudu, K.N. Improved microbial degradation of animal hair waste from leather industry using Brevibacterium luteolum (MTCC 5982). J. Clean. Prod. 2018, 189, 701–708. [Google Scholar] [CrossRef]
- Fagbemi, O.D.; Sithole, B.; Tesfaye, T. Optimization of keratin protein extraction from waste chicken feathers using hybrid pre-treatment techniques. Sustain. Chem. Pharm. 2020, 17, 100267. [Google Scholar] [CrossRef]
- Kumar Kumawat, T.; Sharma, A.; Sharma, V.; Chandra, S. Keratin waste: The biodegradable polymers. In Keratin; Blumenberg, M., Ed.; IntechOpen: London, UK, 2018. [Google Scholar]
- Alexandratos, N.; Bruinsma, J. World Agriculture towards 2030/2050: The 2012 Revision; FAO: Rome, Italy, 2012; p. 154. [Google Scholar]
- Anbu, P.; Hilda, A.; Gopinath, S.C.B. Keratinophilic fungi of poultry farm and feather dumping soil in tamil nadu, india. Mycopathologia 2004, 158, 303–309. [Google Scholar] [CrossRef]
- Duan, Y.; Awasthi, S.K.; Liu, T.; Pandey, A.; Zhang, Z.; Kumar, S.; Awasthi, M.K. Succession of keratin-degrading bacteria and associated health risks during pig manure composting. J. Clean. Prod. 2020, 258, 120624. [Google Scholar] [CrossRef]
- Dou, Y.; Huang, X.; Zhang, B.; He, M.; Yin, G.; Cui, Y. Preparation and characterization of a dialdehyde starch crosslinked feather keratin film for food packaging application. RSC Adv. 2015, 5, 27168–27174. [Google Scholar] [CrossRef]
- Shavandi, A.; Carne, A.; Bekhit, A.A.; Bekhit, A.E.-D.A. An improved method for solubilisation of wool keratin using peracetic acid. J. Environ. Chem. Eng. 2017, 5, 1977–1984. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, R.; Zhao, W. Improving digestibility of feather meal by steam flash explosion. J. Agric. Food Chem. 2014, 62, 2745–2751. [Google Scholar] [CrossRef]
- Tesfaye, T.; Sithole, B.; Ramjugernath, D.; Chunilall, V. Valorisation of chicken feathers: Characterisation of chemical properties. Waste Manag. 2017, 68, 626–635. [Google Scholar] [CrossRef] [PubMed]
- Alahyaribeik, S.; Ullah, A. Methods of keratin extraction from poultry feathers and their effects on antioxidant activity of extracted keratin. Int. J. Biol. Macromol. 2020, 148, 449–456. [Google Scholar] [CrossRef]
- Park, M.; Kim, B.S.; Shin, H.K.; Park, S.J.; Kim, H.Y. Preparation and characterization of keratin-based biocomposite hydrogels prepared by electron beam irradiation. Mater. Sci. Eng. C Mater. Biol. Appl. 2013, 33, 5051–5057. [Google Scholar] [CrossRef]
- Poole, A.J.; Lyons, R.E.; Church, J.S. Dissolving feather keratin using sodium sulfide for bio-polymer applications. J. Polym. Environ. 2011, 19, 995–1004. [Google Scholar] [CrossRef]
- Calin, M.; Raut, I.; Arsene, M.L.; Capra, L.; Gurban, A.M.; Doni, M.; Jecu, L. Applications of fungal strains with keratin-degrading and plant growth promoting characteristics. Agronomy 2019, 9, 543. [Google Scholar] [CrossRef] [Green Version]
- Callegaro, K.; Brandelli, A.; Daroit, D.J. Beyond plucking: Feathers bioprocessing into valuable protein hydrolysates. Waste Manag. 2019, 95, 399–415. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Tonin, C. Characterisation of keratin biomass from butchery and wool industry wastes. J. Mol. Struct. 2009, 938, 35–40. [Google Scholar] [CrossRef]
- de Guzman, R.C.; Merrill, M.R.; Richter, J.R.; Hamzi, R.I.; Greengauz-Roberts, O.K.; Van Dyke, M.E. Mechanical and biological properties of keratose biomaterials. Biomaterials 2011, 32, 8205–8217. [Google Scholar] [CrossRef] [PubMed]
- Saravanan, K.; Dhurai, B. Exploration on the amino acid content and morphological structure in chicken feather fiber. J. Text. Appar. Technol. Manag. 2012, 7, 3. [Google Scholar]
- Grazziotin, A.; Pimentel, F.A.; Sangali, S.; de Jong, E.V.; Brandelli, A. Production of feather protein hydrolysate by keratinolytic bacterium vibrio sp. Kr2. Bioresour. Technol. 2007, 98, 3172–3175. [Google Scholar] [CrossRef]
- Peng, Z.; Mao, X.; Zhang, J.; Du, G.; Chen, J. Biotransformation of keratin waste to amino acids and active peptides based on cell-free catalysis. Biotechnol. Biofuels 2020, 13, 61. [Google Scholar] [CrossRef]
- Feroz, S.; Muhammad, N.; Ranayake, J.; Dias, G. Keratin-based materials for biomedical applications. Bioact. Mater. 2020, 5, 496–509. [Google Scholar] [CrossRef]
- Donato, R.K.; Mija, A. Keratin associations with synthetic, biosynthetic and natural polymers: An extensive review. Polymers 2020, 12, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hill, P.; Brantley, H.; Van Dyke, M. Some properties of keratin biomaterials: Kerateines. Biomaterials 2010, 31, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Jacob, J.T.; Coulombe, P.A.; Kwan, R.; Omary, M.B. Types i and ii keratin intermediate filaments. Cold Spring Harb. Perspect. Biol. 2018, 10, 4. [Google Scholar] [CrossRef] [Green Version]
- Fraser, R.D.B.; MacRae, T.P.; Sparrow, L.G.; Parry, D.A.D. Disulphide bonding in α-keratin. Int. J. Biol. Macromol. 1988, 10, 106–112. [Google Scholar] [CrossRef]
- Fraser, R.D.; Parry, D.A. The structural basis of the filament-matrix texture in the avian/reptilian group of hard beta-keratins. J. Struct. Biol. 2011, 173, 391–405. [Google Scholar] [CrossRef]
- Rajabi, M.; Ali, A.; McConnell, M.; Cabral, J. Keratinous materials: Structures and functions in biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2020, 110, 110612. [Google Scholar] [CrossRef]
- Ghosh, A.; Collie, S.R. Keratinous materials as novel absorbent systems for toxic pollutants. Def. Sci. J. 2014, 64, 209. [Google Scholar] [CrossRef] [Green Version]
- Yamauchi, K.; Yamauchi, A.; Kusunoki, T.; Kohda, A.; Konishi, Y. Preparation of stable aqueous solution of keratins, and physiochemical and biodegradational properties of films. J. Biomed. Mater. Res. 1996, 31, 439–444. [Google Scholar] [CrossRef]
- Poranki, D.; Whitener, W.; Howse, S.; Mesen, T.; Howse, E.; Burnell, J.; Greengauz-Roberts, O.; Molnar, J.; Van Dyke, M. Evaluation of skin regeneration after burns in vivo and rescue of cells after thermal stress in vitro following treatment with a keratin biomaterial. J. Biomater. Appl. 2014, 29, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Conrad, M.; Lemb, K.; Schubert, T.; Markl, J. Biochemical identification and tissue-specific expression patterns of keratins in the zebrafish danio rerio. Cell Tissue Res. 1998, 293, 195–205. [Google Scholar] [CrossRef]
- Shibuya, K.; Tsutsui, S.; Nakamura, O. Fugu, takifugu ruberipes, mucus keratins act as defense molecules against fungi. Mol. Immunol. 2019, 116, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Alibardi, L. Keratinization of sheath and calamus cells in developing and regenerating feathers. Ann. Anat. 2007, 189, 583–595. [Google Scholar] [CrossRef]
- Skieresz-Szewczyk, K.; Jackowiak, H.; Buchwald, T.; Szybowicz, M. Localization of alpha-keratin and beta-keratin (corneous beta protein) in the epithelium on the ventral surface of the lingual apex and its lingual nail in the domestic goose (anser anser f. Domestica) by using immunohistochemistry and raman microspectroscopy analysis. Anat. Rec. 2017, 300, 1361–1368. [Google Scholar]
- Calvaresi, M.; Eckhart, L.; Alibardi, L. The molecular organization of the beta-sheet region in corneous beta-proteins (beta-keratins) of sauropsids explains its stability and polymerization into filaments. J. Struct. Biol. 2016, 194, 282–291. [Google Scholar] [CrossRef]
- Giroud, A.; Leblond, C.P. The keratinization of epidermis and its derivatives, especially the hair, as shown by x-ray diffraction and histochemical studies. Ann. N. Y. Acad. Sci. 1951, 53, 613–626. [Google Scholar] [CrossRef]
- Powell, B.C.; Rogers, G.E. Hard keratin if and associated proteins. In Cellular and Molecular Biology of Intermediate Filaments; Goldman, R.D., Steinert, P.M., Eds.; Springer: Boston, MA, USA, 1990. [Google Scholar]
- Fraser, R.D.B.; MacRae, T.P. Molecular structure and mechanical properties of keratins. In The Mechanical Properties of Biological Materials; Currey, J.F.V.A.D., Ed.; Cambridge University Press: Cambridge, UK, 1980; pp. 211–246. [Google Scholar]
- Mercer, E.H. Keratin and Keratinization; Pergamon Press: Oxford, UK, 1961. [Google Scholar]
- Fraser, R.D.B.; MacRae, T.P.; Rogers, G.E. Keratins: Their Composition, Structure and Biosynthesis; The University of Chicago Press: Springfield, IL, USA, 1972. [Google Scholar]
- Ramakrishnan, N.; Sharma, S.; Gupta, A.; Alashwal, B.Y. Keratin based bioplastic film from chicken feathers and its characterization. Int. J. Biol. Macromol. 2018, 111, 352–358. [Google Scholar] [CrossRef]
- Szewciw, L.J.; de Kerckhove, D.G.; Grime, G.W.; Fudge, D.S. Calcification provides mechanical reinforcement to whale baleen alpha-keratin. Proc. Biol. Sci. 2010, 277, 2597–2605. [Google Scholar]
- Agrahari, S.; Wadhwa, N. Degradation of chicken feather a poultry waste product by keratinolytic bacteria isolated from dumping site at ghazipur poultry processing plant. Int. J. Poult. Sci. 2010, 9, 482–489. [Google Scholar] [CrossRef] [Green Version]
- Paul, T.; Halder, S.K.; Das, A.; Bera, S.; Maity, C.; Mandal, A.; Das, P.S.; Mohapatra, P.K.D.; Pati, B.R.; Mondal, K.C. Exploitation of chicken feather waste as a plant growth promoting agent using keratinase producing novel isolate paenibacillus woosongensis tkb2. Biocatal. Agric. Biotechnol. 2013, 2, 50–57. [Google Scholar] [CrossRef]
- Ullah, A.; Vasanthan, T.; Bressler, D.; Elias, A.L.; Wu, J. Bioplastics from feather quill. Biomacromolecules 2011, 12, 3826–3832. [Google Scholar] [CrossRef]
- Kao, W.W. Keratin expression by corneal and limbal stem cells during development. Exp. Eye Res. 2020, 200, 108206. [Google Scholar] [CrossRef]
- Abebe, T. Extraction and Optimization of Natural Protein (Keratin) from Waste Chicken Feather for the Development of Anti-Ageing Cream; Addis Ababa University: Addis Ababa, Ethiopia, 2017. [Google Scholar]
- Wang, K.; Li, R.; Ma, J.H.; Jian, Y.K.; Che, J.N. Extracting keratin from wool by using l-cysteine. Green Chem. 2016, 18, 476–481. [Google Scholar] [CrossRef]
- Zoccola, M.; Aluigi, A.; Patrucco, A.; Vineis, C.; Forlini, F.; Locatelli, P.; Sacchi, M.C.; Tonin, C. Microwave-assisted chemical-free hydrolysis of wool keratin. Text. Res. J. 2012, 82, 2006–2018. [Google Scholar] [CrossRef]
- Vineis, C.; Varesano, A.; Varchi, G.; Aluigi, A. Extraction and characterization of keratin from different biomasses. In Keratin as a Protein Biopolymer; Springer Series on Polymer and Composite Materials; Sharma, S., Ashok, K., Eds.; Springer: Cham, Switzerland, 2019. [Google Scholar]
- Saha, S.; Arshad, M.; Zubair, M.; Ullah, A. Keratin as a Biopolymer; Springer: Cham, Switzerland, 2019; pp. 163–185. [Google Scholar]
- Wang, D.; Yang, X.H.; Tang, R.C.; Yao, F. Extraction of keratin from rabbit hair by a deep eutectic solvent and its characterization. Polymers 2018, 10, 993. [Google Scholar] [CrossRef] [Green Version]
- Ding, S.; Sun, Y.; Chen, H.; Xu, C.; Hu, Y. An ultrasonic-ionic liquid process for the efficient acid catalyzed hydrolysis of feather keratin. Chin. J. Chem. Eng. 2019, 27, 660–667. [Google Scholar] [CrossRef]
- Earland, C.; Knight, C.S. Studies on the structure of keratin: I. The analysis of fractions isolated from wool oxidized with peracetic acid. Biochim. Biophys. Acta 1955, 17, 457–461. [Google Scholar] [CrossRef]
- Buchanan, J.H. A cystine-rich protein fraction from oxidized α-keratin. Biochem. J. 1977, 167, 489–491. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, A.; Clerens, S.; Deb-Choudhury, S.; Dyer, J.M. Thermal effects of ionic liquid dissolution on the structures and properties of regenerated wool keratin. Polym. Degrad. Stab. 2014, 108, 108–115. [Google Scholar] [CrossRef]
- Shavandi, A.; Silva, T.H.; Bekhit, A.A.; Bekhit, A.E.A. Keratin: Dissolution, extraction and biomedical application. Biomater. Sci. 2017, 5, 1699–1735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajabinejad, H.; Patrucco, A.; Caringella, R.; Montarsolo, A.; Zoccola, M.; Pozzo, P.D. Preparation of keratin-based microcapsules for encapsulation of hydrophilic molecules. Ultrason. Sonochem. 2018, 40, 527–532. [Google Scholar] [CrossRef]
- Brown, E.M.; Pandya, K.; Taylor, M.M.; Liu, C.-K. Comparison of methods for extraction of keratin from waste wool. Agric. Sci. 2016, 7, 670–679. [Google Scholar] [CrossRef] [Green Version]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Chen, Y.; Ferri, A.; Muresan, A.; Tonin, C. Fabrication and properties of keratoses/polyvinyl alcohol blend films. Polym. Bull. 2020, 77, 3033–3046. [Google Scholar] [CrossRef]
- Agarwal, V.; Panicker, A.G.; Indrakumar, S.; Chatterjee, K. Comparative study of keratin extraction from human hair. Int. J. Biol. Macromol. 2019, 133, 382–390. [Google Scholar] [CrossRef] [PubMed]
- Atri, H.; Bidram, E.; Dunstan, D.E. Reconstituted keratin biomaterial with enhanced ductility. Materials 2015, 8, 7472–7485. [Google Scholar] [CrossRef] [Green Version]
- Khumalo, M.; Sithole, B.; Tesfaye, T. Valorisation of waste chicken feathers: Optimisation of keratin extraction from waste chicken feathers by sodium bisulphite, sodium dodecyl sulphate and urea. J. Environ. Manag. 2020, 262, 110329. [Google Scholar] [CrossRef]
- Schrooyen, P.M.M.; Dijkstra, P.J.; Oberthür, R.C.; Jan Feijen, A.B. Stabilization of solutions of feather keratins by sodium dodecyl sulfate. J. Colloid Interface Sci. 2001, 240, 30–39. [Google Scholar] [CrossRef] [PubMed]
- Deb-Choudhury, S.; Plowman, J.E.; Harland, D.P. Isolation and analysis of keratins and keratin-associated proteins from hair and wool. Methods Enzymol. 2016, 568, 279–301. [Google Scholar] [PubMed]
- Rajabinejad, H.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Physicochemical properties of keratin extracted from wool by various methods. Text. Res. J. 2017, 88, 2415–2424. [Google Scholar] [CrossRef]
- Ramya, K.R.; Thangam, R.; Madhan, B. Comparative analysis of the chemical treatments used in keratin extraction from red sheep’s hair and the cell viability evaluations of this keratin for tissue engineering applications. Process Biochem. 2020, 90, 223–232. [Google Scholar] [CrossRef]
- Sinkiewicz, I.; Śliwińska, A.; Staroszczyk, H.; Kołodziejska, I. Alternative methods of preparation of soluble keratin from chicken feathers. Waste Biomass Valoriz. 2016, 8, 1043–1048. [Google Scholar] [CrossRef]
- Isarankura Na Ayutthaya, S.; Tanpichai, S.; Wootthikanokkhan, J. Keratin extracted from chicken feather waste: Extraction, preparation, and structural characterization of the keratin and keratin/biopolymer films and electrospuns. J. Polym. Environ. 2015, 23, 506–516. [Google Scholar] [CrossRef]
- Bertini, F.; Canetti, M.; Patrucco, A.; Zoccola, M. Wool keratin-polypropylene composites: Properties and thermal degradation. Polym. Degrad. Stab. 2013, 98, 980–987. [Google Scholar] [CrossRef]
- Wang, X.; Shi, Z.; Zhao, Q.; Yun, Y. Study on the structure and properties of biofunctional keratin from rabbit hair. Materials 2021, 14, 379. [Google Scholar] [CrossRef]
- Holkar, C.R.; Jain, S.S.; Jadhav, A.J.; Pinjari, D.V. Valorization of keratin based waste. Process Saf. Environ. Prot. 2018, 115, 85–98. [Google Scholar] [CrossRef]
- Bhavsar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Rovero, G.; Tonin, C. Comparative study on the effects of superheated water and high temperature alkaline hydrolysis on wool keratin. Text. Res. J. 2016, 87, 1696–1705. [Google Scholar] [CrossRef]
- Gough, C.R.; Rivera-Galletti, A.; Cowan, D.A.; Salas-de la Cruz, D.; Hu, X. Protein and polysaccharide-based fiber materials generated from ionic liquids: A review. Molecules 2020, 25, 3362. [Google Scholar] [CrossRef]
- Han, D.; Row, K.H. Recent applications of ionic liquids in separation technology. Molecules 2010, 15, 2405–2426. [Google Scholar] [CrossRef] [Green Version]
- Isik, M.; Sardon, H.; Mecerreyes, D. Ionic liquids and cellulose: Dissolution, chemical modification and preparation of new cellulosic materials. Int. J. Mol. Sci. 2014, 15, 11922–11940. [Google Scholar] [CrossRef] [PubMed]
- Sowmiah, S.; Srinivasadesikan, V.; Tseng, M.C.; Chu, Y.H. On the chemical stabilities of ionic liquids. Molecules 2009, 14, 3780–3813. [Google Scholar] [CrossRef] [Green Version]
- Ventura, S.P.M.; FA, E.S.; Quental, M.V.; Mondal, D.; Freire, M.G.; Coutinho, J.A.P. Ionic-liquid-mediated extraction and separation processes for bioactive compounds: Past, present, and future trends. Chem. Rev. 2017, 117, 6984–7052. [Google Scholar] [CrossRef] [PubMed]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Fredericks, D.; Patti, A.F.; MacFarlane, D.R. Dissolution of feather keratin in ionic liquids. Green Chem. 2013, 15, 525. [Google Scholar] [CrossRef]
- Idris, A.; Vijayaraghavan, R.; Rana, U.A.; Patti, A.F.; MacFarlane, D.R. Dissolution and regeneration of wool keratin in ionic liquids. Green Chem. 2014, 16, 2857–2864. [Google Scholar] [CrossRef]
- Ji, Y.; Chen, J.; Lv, J.; Li, Z.; Xing, L.; Ding, S. Extraction of keratin with ionic liquids from poultry feather. Sep. Purif. Technol. 2014, 132, 577–583. [Google Scholar] [CrossRef]
- Liu, X.; Nie, Y.; Meng, X.; Zhang, Z.; Zhang, X.; Zhang, S. Dbn-based ionic liquids with high capability for the dissolution of wool keratin. RSC Adv. 2017, 7, 1981–1988. [Google Scholar] [CrossRef] [Green Version]
- Azmi, N.A.; Idris, A.; Yusof, N.S.M. Ultrasonic technology for value added products from feather keratin. Ultrason. Sonochem. 2018, 47, 99–107. [Google Scholar] [CrossRef]
- Chen, J.; Li, Y.; Wang, X.; Liu, W. Application of deep eutectic solvents in food analysis: A review. Molecules 2019, 24, 4594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dai, Y.; Row, K.H. Application of natural deep eutectic solvents in the extraction of quercetin from vegetables. Molecules 2019, 24, 2300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jablonský, M.; Majová, V.; Šima, J.; Hroboňová, K.; Lomenová, A. Involvement of deep eutectic solvents in extraction by molecularly imprinted polymers—A minireview. Crystals 2020, 10, 217. [Google Scholar] [CrossRef] [Green Version]
- Sakhno, T.V.; Barashkov, N.N.; Irgibaeva, I.S.; Mendigaliyeva, S.; Bostan, D.S. Ionic liquids and deep eutectic solvents and their use for dissolving animal hair. Adv. Chem. Eng. Sci. 2020, 10, 40–51. [Google Scholar] [CrossRef] [Green Version]
- Kornillowicz-Kowalska, T.; Bohacz, J. Biodegradation of keratin waste: Theory and practical aspects. Waste Manag. 2011, 31, 1689–1701. [Google Scholar] [CrossRef] [PubMed]
- De Oliveira Martinez, J.P.; Cai, G.; Nachtschatt, M.; Navone, L.; Zhang, Z.; Robins, K.; Speight, R. Challenges and opportunities in identifying and characterising keratinases for value-added peptide production. Catalysts 2020, 10, 184. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.; Ramnani, P. Microbial keratinases and their prospective applications: An overview. Appl. Microbiol. Biotechnol. 2006, 70, 21–33. [Google Scholar] [CrossRef]
- Purchase, D. Microbial keratinases: Characteristics, biotechnological applications and potential. In The Handbook of Microbial Bioresources; Gupta, V.K., Sharma, G.D., Tuohy, M.G., Gaur, R., Eds.; CABI Publishing: Oxfordshire, UK, 2016; pp. 638–675. [Google Scholar]
- Vidmar, B.; Vodovnik, M. Microbial keratinases: Enzymes with promising biotechnological applications. Food Technol. Biotechnol. 2018, 56, 312–328. [Google Scholar] [CrossRef] [PubMed]
- Su, C.; Gong, J.S.; Qin, J.; Li, H.; Li, H.; Xu, Z.H.; Shi, J.S. The tale of a versatile enzyme: Molecular insights into keratinase for its industrial dissemination. Biotechnol. Adv. 2020, 45, 107655. [Google Scholar] [CrossRef] [PubMed]
- Villa, A.L.V.; Aragão, M.R.S.; dos Santos, E.P.E.A. Feather keratin hydrolysates obtained from microbial keratinases: Effect on hair fiber. BMC Biotechnol. 2013, 13, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lo, W.H.; Too, J.R.; Wu, J.Y. Production of keratinolytic enzyme by an indigenous feather-degrading strain bacillus cereus wu2. J. Biosci. Bioeng. 2012, 114, 640–647. [Google Scholar] [CrossRef]
- Nnolim, N.E.; Okoh, A.I.; Nwodo, U.U. Bacillus sp. Fpf-1 produced keratinase with high potential for chicken feather degradation. Molecules 2020, 25, 1505. [Google Scholar] [CrossRef] [Green Version]
- Kumar, A.G.; Swarnalatha, S.; Gayathri, S.; Nagesh, N.; Sekaran, G. Characterization of an alkaline active-thiol forming extracellular serine keratinase by the newly isolated bacillus pumilus. J. Appl. Microbiol. 2008, 104, 411–419. [Google Scholar] [CrossRef]
- Jaouadi, N.Z.; Rekik, H.; Badis, A.; Trabelsi, S.; Belhoul, M.; Yahiaoui, A.B.; Ben Aicha, H.; Toumi, A.; Bejar, S.; Jaouadi, B. Biochemical and molecular characterization of a serine keratinase from brevibacillus brevis us575 with promising keratin-biodegradation and hide-dehairing activities. PLoS ONE 2013, 8, e76722. [Google Scholar] [CrossRef] [PubMed]
- Kuo, J.-M.; Yang, J.-I.; Chen, W.-M.; Pan, M.-H.; Tsai, M.-L.; Lai, Y.-J.; Hwang, A.; Pan, B.S.; Lin, C.-Y. Purification and characterization of a thermostable keratinase from meiothermus sp. I40. Int. Biodeterior. Biodegrad. 2012, 70, 111–116. [Google Scholar] [CrossRef]
- Fang, Z.; Zhang, J.; Liu, B.; Du, G.; Chen, J. Biochemical characterization of three keratinolytic enzymes from stenotrophomonas maltophilia bbe11-1 for biodegrading keratin wastes. Int. Biodeterior. Biodegrad. 2013, 82, 166–172. [Google Scholar] [CrossRef]
- Syed, D.G.; Lee, J.C.; Li, W.J.; Kim, C.J.; Agasar, D. Production, characterization and application of keratinase from streptomyces gulbargensis. Bioresour. Technol. 2009, 100, 1868–1871. [Google Scholar] [CrossRef]
- Bohacz, J. Biodegradation of feather waste keratin by a keratinolytic soil fungus of the genus chrysosporium and statistical optimization of feather mass loss. World J. Microbiol. Biotechnol. 2017, 33, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazotto, A.M.; Couri, S.; Damaso, M.C.T.; Vermelho, A.B. Degradation of feather waste by aspergillus niger keratinases: Comparison of submerged and solid-state fermentation. Int. Biodeterior. Biodegrad. 2013, 85, 189–195. [Google Scholar] [CrossRef] [Green Version]
- Cavello, I.A.; Hours, R.A.; Rojas, N.L.; Cavalitto, S.F. Purification and characterization of a keratinolytic serine protease from purpureocillium lilacinum lps # 876. Process Biochem. 2013, 48, 972–978. [Google Scholar]
- Anbu, P.; Hilda, A.; Sur, H.-W.; Hur, B.-K.; Jayanthi, S. Extracellular keratinase from trichophyton sp. Ha-2 isolated from feather dumping soil. Int. Biodeterior. Biodegrad. 2008, 62, 287–292. [Google Scholar] [CrossRef]
- Călin, M.; Constantinescu-Aruxandei, D.; Alexandrescu, E.; Răut, I.; Doni, M.B.; Arsene, M.-L.; Oancea, F.; Jecu, L.; Lazăr, V. Degradation of keratin substrates by keratinolytic fungi. Electron. J. Biotechnol. 2017, 28, 101–112. [Google Scholar] [CrossRef]
- Lange, L.; Huang, Y.; Busk, P.K. Microbial decomposition of keratin in nature—A new hypothesis of industrial relevance. Appl. Microbiol. Biotechnol. 2016, 100, 2083–2096. [Google Scholar] [CrossRef] [Green Version]
- Kadić, A.; Várnai, A.; Eijsink, V.G.; Horn, S.J.; Lidén, G. In situ measurements of oxidation–reduction potential and hydrogen peroxide concentration as tools for revealing lpmo inactivation during enzymatic saccharification of cellulose. Biotechnol. Biofuels 2021, 14, 1–10. [Google Scholar] [CrossRef]
- Xu, W.; Ke, G.; Wu, J.; Wang, X. Modification of wool fiber using steam explosion. Eur. Polym. J. 2006, 42, 2168–2173. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Zhao, W.; Yang, R. Steam flash explosion assisted dissolution of keratin from feathers. ACS Sustain. Chem. Eng. 2015, 3, 2036–2042. [Google Scholar] [CrossRef]
- Guo, L.; Lu, L.; Yin, M.; Yang, R.; Zhang, Z.; Zhao, W. Valorization of refractory keratinous waste using a new and sustainable bio-catalysis. Chem. Eng. J. 2020, 397, 125420. [Google Scholar] [CrossRef]
- Lee, Y.S.; Phang, L.-Y.; Ahmad, S.A.; Ooi, P.T. Microwave-alkali treatment of chicken feathers for protein hydrolysate production. Waste Biomass Valoriz. 2016, 7, 1147–1157. [Google Scholar] [CrossRef]
- Tasaki, K. A novel thermal hydrolysis process for extraction of keratin from hog hair for commercial applications. Waste Manag. 2020, 104, 33–41. [Google Scholar] [CrossRef]
- Barba, C.; Mendez, S.; Roddick-Lanzilotta, A.; Kelly, R.; Parra, J.L.; Coderch, L. Cosmetic effectiveness of topically applied hydrolysed keratin peptides and lipids derived from wool. Skin Res. Technol. 2008, 14, 243–248. [Google Scholar] [CrossRef]
- Venkataramani, D.; Tsulaia, A.; Amin, S. Fundamentals and applications of particle stabilized emulsions in cosmetic formulations. Adv. Colloid Interface Sci. 2020, 283, 102234. [Google Scholar] [CrossRef] [PubMed]
- Kakkar, P.; Madhan, B. Fabrication of keratin-silica hydrogel for biomedical applications. Mater. Sci. Eng. C Mater. Biol. Appl. 2016, 66, 178–184. [Google Scholar] [CrossRef] [PubMed]
- Mi, X.; Xu, H.; Yang, Y. Submicron amino acid particles reinforced 100% keratin biomedical films with enhanced wet properties via interfacial strengthening. Colloids Surf. B Biointerfaces 2019, 177, 33–40. [Google Scholar] [CrossRef]
- Patrucco, A.; Visai, L.; Fassina, L.; Magenes, G.; Tonin, C. Keratin-based matrices from wool fibers and human hair. In Materials for Biomedical Engineering; Grumezescu, V., Grumezescu, A.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 375–403. [Google Scholar]
- Posati, T.; Giuri, D.; Nocchetti, M.; Sagnella, A.; Gariboldi, M.; Ferroni, C.; Sotgiu, G.; Varchi, G.; Zamboni, R.; Aluigi, A. Keratin-hydrotalcites hybrid films for drug delivery applications. Eur. Polym. J. 2018, 105, 177–185. [Google Scholar] [CrossRef]
- Ye, J.P.; Gong, J.S.; Su, C.; Liu, Y.G.; Jiang, M.; Pan, H.; Li, R.Y.; Geng, Y.; Xu, Z.H.; Shi, J.S. Fabrication and characterization of high molecular keratin based nanofibrous membranes for wound healing. Colloids Surf. B Biointerfaces 2020, 194, 111158. [Google Scholar] [CrossRef]
- Yin, X.C.; Li, F.Y.; He, Y.F.; Wang, Y.; Wang, R.M. Study on effective extraction of chicken feather keratins and their films for controlling drug release. Biomater. Sci. 2013, 1, 528–536. [Google Scholar] [CrossRef]
- Chen, Y.; Li, W.; Zhang, S. A multifunctional eco-friendly fertilizer used keratin-based superabsorbent as coatings for slow-release urea and remediation of contaminated soil. Prog. Org. Coat. 2021, 154, 106158. [Google Scholar] [CrossRef]
- Li, W.; Chen, Y.; Zhang, S. Hydrolysis of abandoned bovine hair by pulping spent liquor and preparation of degradable keratin-based sprayable mulch film. BioResources 2020, 15, 5058–5071. [Google Scholar]
- Faraon, V.A.; Neamtu, C.; Oancea, F. Npk fertilizers’ coatings using biodegradable by-products from the agro-food industry. Proceedings 2019, 29, 110. [Google Scholar] [CrossRef] [Green Version]
- Evangelou, M.W.H.; Ebel, M.; Koerner, A.; Schaeffer, A. Hydrolysed wool: A novel chelating agent for metal chelant-assisted phytoextraction from soil. Chemosphere 2008, 72, 525–531. [Google Scholar] [CrossRef]
- Shavandi, A.; Bekhit, A.E.-D.A.; Carne, A.; Bekhit, A. Evaluation of keratin extraction from wool by chemical methods for bio-polymer application. J. Bioact. Compat. Polym. 2016, 32, 163–177. [Google Scholar] [CrossRef]
- Lee, H.; Noh, K.; Lee, S.C.; Kwon, I.-K.; Han, D.-W.; Lee, I.-S.; Hwang, Y.-S. Human hair keratin and its-based biomaterials for biomedical applications. Tissue Eng. Regen. Med. 2014, 11, 255–265. [Google Scholar] [CrossRef]
- Zhai, M.; Xu, Y.; Zhou, B.; Jing, W. Keratin-chitosan/n-zno nanocomposite hydrogel for antimicrobial treatment of burn wound healing: Characterization and biomedical application. J. Photochem. Photobiol. B 2018, 180, 253–258. [Google Scholar] [CrossRef] [PubMed]
- Deng, X.; Gould, M.; Ali, M.A. Fabrication and characterisation of melt-extruded chitosan/keratin/pcl/peg drug-eluting sutures designed for wound healing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 120, 111696. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Park, B.J.; Lee, Y.; Park, N.J.; Park, K.M.; Hwang, Y.-S.; Park, K.D. Human hair keratin-based hydrogels as dynamic matrices for facilitating wound healing. J. Ind. Eng. Chem. 2019, 73, 142–151. [Google Scholar] [CrossRef]
- Su, S.; Bedir, T.; Kalkandelen, C.; Ozan Başar, A.; Turkoğlu Şaşmazel, H.; Bulent Ustundag, C.; Sengor, M.; Gunduz, O. Coaxial and emulsion electrospinning of extracted hyaluronic acid and keratin based nanofibers for wound healing applications. Eur. Polym. J. 2021, 142, 110158. [Google Scholar] [CrossRef]
- Rajabinejad, H.; Bucişcanu, I.-I.; Maier, S.S. Current approaches for raw wool waste management and unconventional valorization: A review. Environ. Eng. Manag. J. 2019, 18, 1439–1456. [Google Scholar]
- Yeo, I.; Lee, Y.J.; Song, K.; Jin, H.S.; Lee, J.E.; Kim, D.; Lee, D.W.; Kang, N.J. Low-molecular weight keratins with anti-skin aging activity produced by anaerobic digestion of poultry feathers with fervidobacterium islandicum aw-1. J. Biotechnol. 2018, 271, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Zahara, I.; Arshad, M.; Naeth, M.A.; Siddique, T.; Ullah, A. Feather keratin derived sorbents for the treatment of wastewater produced during energy generation processes. Chemosphere 2020, 273, 128545. [Google Scholar] [CrossRef]
- Jin, X.; Wang, H.; Jin, X.; Wang, H.; Chen, L.; Wang, W.; Lin, T.; Zhu, Z. Preparation of keratin/pet nanofiber membrane and its high adsorption performance of cr(vi). Sci. Total Environ. 2020, 710, 135546. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Varghese, N.; Dasgupta, S.; Sood, A.K.; Chatterjee, K. Engineering a 3d mos2 foam using keratin exfoliated nanosheets. Chem. Eng. J. 2019, 374, 254–262. [Google Scholar] [CrossRef] [Green Version]
- Miranda-Vilela, A.L.; Botelho, A.J.; Muehlmann, L.A. An overview of chemical straightening of human hair: Technical aspects, potential risks to hair fibre and health and legal issues. Int. J. Cosmet. Sci. 2014, 36, 2–11. [Google Scholar] [CrossRef] [Green Version]
- Tinoco, A.; Gonçalves, F.; Costa, A.F.; Freitas, D.S.; Cavaco-Paulo, A.; Ribeiro, A. Keratin: Zein particles as vehicles for fragrance release on hair. Ind. Crop. Prod. 2021, 159, 113067. [Google Scholar] [CrossRef]
- Guidotti, G.; Soccio, M.; Posati, T.; Sotgiu, G.; Tiboni, M.; Barbalinardo, M.; Valle, F.; Casettari, L.; Zamboni, R.; Lotti, N.; et al. Regenerated wool keratin-polybutylene succinate nanofibrous mats for drug delivery and cells culture. Polym. Degrad. Stab. 2020, 179, 109272. [Google Scholar] [CrossRef]
- Song, K.; Qian, X.; Zhu, X.; Li, X.; Hong, X. Fabrication of mechanical robust keratin film by mesoscopic molecular network reconstruction and its performance for dye removal. J. Colloid Interface Sci. 2020, 579, 28–36. [Google Scholar] [CrossRef] [PubMed]
- Adler, S.A.; Slizyte, R.; Honkapää, K.; Løes, A.-K. In vitro pepsin digestibility and amino acid composition in soluble and residual fractions of hydrolyzed chicken feathers. Poult. Sci. 2018, 97, 3343–3357. [Google Scholar] [CrossRef]
- Fakhfakh, N.; Ktari, N.; Siala, R.; Nasri, M. Wool-waste valorization: Production of protein hydrolysate with high antioxidative potential by fermentation with a new keratinolytic bacterium, b acillus pumilus a1. J. Appl. Microbiol. 2013, 115, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Kersanté, P.; Le Reste, G.; Diringer, B.; Quimi, J.; Sergheraert, R.; Duperray, J. Free amino acids mix made of poultry keratin improves survival of whiteleg shrimp post larvae (litopenaeus vannamei) challenged with acute hepatopancreatic necrosis disease and white spot syndrome virus. Aquac. Int. 2021, 29, 879–890. [Google Scholar] [CrossRef]
- Bhaysar, P.; Zoccola, M.; Patrucco, A.; Montarsolo, A.; Mossotti, R.; Rovero, G.; Giansetti, M.; Tonin, C. Superheated water hydrolysis of waste wool in a semi-industrial reactor to obtain nitrogen fertilizers. ACS Sustain. Chem. Eng. 2016, 4, 6722–6731. [Google Scholar] [CrossRef]
- Choi, J.-M.; Nelson, P.V. Developing a slow-release nitrogen fertilizer from organic sources: Ii. Using poultry feathers. J. Am. Soc. Hortic. Sci. 1996, 121, 634–638. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.C.; Tong, Z.H.; Geng, Y.Q.; Li, Y.C.; Zhang, M. Biobased polymer composites derived from corn stover and feather meals as double-coating materials for controlled-release and water-retention urea fertilizers. J. Agric. Food Chem. 2013, 61, 8166–8174. [Google Scholar] [CrossRef]
- Mihăilă, E.-G.; Faraon, V.A.; Deșliu-Avram, M.; Neamțu, C.; Constantinescu-Aruxandei, D.; Oancea, F. New controlled-release fertilizers with keratin-based coating from chicken waste feathers. Proceedings 2020, 57, 20. [Google Scholar] [CrossRef]
- Gurav, R.G.; Jadhav, J.P. A novel source of biofertilizer from feather biomass for banana cultivation. Environ. Sci. Pollut. Res. 2013, 20, 4532–4539. [Google Scholar] [CrossRef] [PubMed]
- Kaur, M.; Bhari, R.; Singh, R.S. Chicken feather waste-derived protein hydrolysate as a potential biostimulant for cultivation of mung beans. Biologia 2021, 76, 1807–1815. [Google Scholar] [CrossRef]
- Zoccola, M.; Montarsolo, A.; Mossotti, R.; Patrucco, A.; Tonin, C. Green hydrolysis as an emerging technology to turn wool waste into organic nitrogen fertilizer. Waste Biomass Valoriz. 2015, 6, 891–897. [Google Scholar] [CrossRef]
- Popko, M.; Michalak, I.; Wilk, R.; Gramza, M.; Chojnacka, K.; Górecki, H. Effect of the new plant growth biostimulants based on amino acids on yield and grain quality of winter wheat. Molecules 2018, 23, 470. [Google Scholar] [CrossRef] [Green Version]
- Filipović, V.; Bristow, K.L.; Filipović, L.; Wang, Y.; Sintim, H.Y.; Flury, M.; Šimůnek, J. Sprayable biodegradable polymer membrane technology for cropping systems: Challenges and opportunities. Environ. Sci. Technol. 2020, 54, 4709–4711. [Google Scholar] [CrossRef] [Green Version]
- Braunack, M.V.; Zaja, A.; Tam, K.; Filipović, L.; Filipović, V.; Wang, Y.; Bristow, K.L. A sprayable biodegradable polymer membrane (sbpm) technology: Effect of band width and application rate on water conservation and seedling emergence. Agric. Water Manag. 2020, 230, 105900. [Google Scholar] [CrossRef]
- Giaccone, M.; Cirillo, C.; Scognamiglio, P.; Teobaldelli, M.; Mataffo, A.; Stinca, A.; Pannico, A.; Immirzi, B.; Santagata, G.; Malinconico, M. Biodegradable mulching spray for weed control in the cultivation of containerized ornamental shrubs. Chem. Biol. Technol. Agric. 2018, 5, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.J.; Cardamone, J.M.; Irwin, P.L.; Brown, E.M. Keratin capped silver nanoparticles–synthesis and characterization of a nanomaterial with desirable handling properties. Colloids Surf. B Biointerfaces 2011, 88, 354–361. [Google Scholar] [CrossRef]
- Kalishwaralal, K.; Jeyabharathi, S.; Sundar, K.; Muthukumaran, A. Sodium selenite/selenium nanoparticles (senps) protect cardiomyoblasts and zebrafish embryos against ethanol induced oxidative stress. J. Trace Elem. Med. Biol. 2015, 32, 135–144. [Google Scholar] [CrossRef]
- Arnold, R.; Bartl, A.-M.; Hufnagl, E. Production of cord and narrow fabric products with kemafil technology. Band-und Flechtind. 1993, 30, 76–81. [Google Scholar]
- Broda, J.; Gawlowski, A.; Laszczak, R.; Mitka, A.; Przybylo, S.; Grzybowska-Pietras, J.; Rom, M. Application of innovative meandrically arranged geotextiles for the protection of drainage ditches in the clay ground. Geotext. Geomembr. 2017, 45, 45–53. [Google Scholar] [CrossRef]
- Nguyen, G.; Grzybowska-Pietras, J.; Broda, J. Application of innovative ropes from textile waste as an anti-erosion measure. Materials 2021, 14, 1179. [Google Scholar] [CrossRef] [PubMed]
- Marchelli, F.; Rovero, G.; Curti, M.; Arato, E.; Bosio, B.; Moliner, C. An integrated approach to convert lignocellulosic and wool residues into balanced fertilisers. Energies 2021, 14, 497. [Google Scholar] [CrossRef]
- Oancea, F.; Calin, M.; Constantinescu-Aruxandei, D.; Răut, I.; Doni, M.; Arsene, M.L.; Jecu, L. Plant Biostimulant Composition Based on Wool and Process Theroff; RO133240 A2; Romanian State Office for Inventions and Trademark: Bucharest, Romania, 2019. [Google Scholar]
- Andreo-Jimenez, B.; Schilder, M.T.; Nijhuis, E.H.; Te Beest, D.E.; Bloem, J.; Visser, J.H.; van Os, G.; Brolsma, K.; de Boer, W.; Postma, J. Chitin-and keratin-rich soil amendments suppress rhizoctonia solani disease via changes to the soil microbial community. Appl. Environ. Microbiol. 2021, 87, e00318–e00321. [Google Scholar] [CrossRef]




| Property | α-Keratin | β-Keratin |
|---|---|---|
| Filaments’ diameters | IFs: ~7 nm | β-keratin filaments: 3–4 nm |
| Constitutive proteins | IFs: several low-sulfur proteins Matrix: high-sulfur and high-glycine-tyrosine proteins | Presents no different types of proteins The filament and matrix are integrated into one single protein |
| Specific structure | Based on α-helical conformation | Based on β-plated sheet arrangement |
| Molecular weight | 40–68 kDa | 10–22 kDa |
| Keratin Source | Processing Parameters | Reference | |
|---|---|---|---|
| Oxidative Agent | Conditions | ||
| Wool | Peracetic acid 2% | 37 °C; overnight, 180 rpm | [74] |
| Wool | Solid sodium percarbonate 4.5% mass ratio Fiber, liquid ratio 1:35 NaOH 30% | Room temperature; 3–4 h stirring | [74] |
| Wool | Peracetic acid 24% Fiber, liquid ratio 1:60 | 2 days Room temperature Yield 57% | [23] |
| Wool | Peracetic acid 2% Fiber, liquid ratio 1:50 | 25 °C; 24 h | [75] |
| Human hair | Performic acid (100 volume H202/98% formic acid (1:39 v/v) | 4 °C; 18 h | [70] |
| Human hair | Peracetic acid 2% Hair mass, liquid ratio 1:20 | 37 °C; 10 h, 150 rpm | [32] |
| Human hair | Peracetic acid 2.5% | Room temperature; Overnight | [76] |
| Human hair | Thioglycolic acid 0.5 M; | 37 °C; 15 h; pH 10.4 | [76] |
| Keratin Source | Processing Parameters | Reference | |
|---|---|---|---|
| Reductive Agent | Conditions | ||
| Wool | Extractive liquor: thiourea 2.6 M; urea 5 M; 2-mercaptoethanol 5% | 50 °C; 3 days; pH 8.5, 100 rpm | [74] |
| Wool | Extractive liquor: urea 8 M; SDS 0.25%; 2 g sodium metabisulfite Fiber to liquid ratio > 1:70 | 65 °C; 5 h, 120 rpm | [74] |
| Wool | Extractive liquor: urea 8 M; Tris * 0.5 M; DTT **, 0.14 M; ethylenediaminetetraacetic acid EDTA, 6 mM. Fiber to liquid ratio 1:25 | 25 °C; 2.5 h; pH 8.6 | [81] |
| Feathers | Extractive liquor: sodium hydroxide 1.78%; sodium bisulfite 0.5% Fiber to liquid ratio 1:5 | 87 °C; 111 min Yield 68.2 | [17] |
| Wool (red sheep hair) | Extractive liquor: urea 8 M; SDS 0.26 M; mercaptoethanol 1.66 M Fiber to liquid ratio 1:20 | 50 °C; 12 h, under shaking | [82] |
| Wool | Extractive liquor: urea 7 M, thiourea 2 M, Tris 50 mM, TCEP 50 mM Fiber to liquid ratio 10:1 | 18 h; pH 4.3 | [80] |
| Keratin Source | Processing Parameters | Reference | |
|---|---|---|---|
| Extracting Solution | Conditions | ||
| Chicken Feather | Extractive liquor: sodium metabisulfite 0.2 M; urea 8 M; SDS 0.6 g/g feather Fiber to liquid ratio 1:35 | 65 °C; 5 h; pH 6.5 Yield 87.6% | [84] |
| Hair | Sodium sulfide 0.125 M | 40 °C; 4.5 h | [76] |
| Wool | Extractive liquor: urea 8 M; sodium metabisulfite 0.5 M Fiber to liquid ratio 1:25 | 65 °C; 2.5 h; pH 7; | [81] |
| Wool (red sheep hair) | Extractive liquor: urea 8 M; sodium metabisulfite 0.5 M Fiber to liquid ratio 1:20 | 65 °C; 2 h constant stirring | [82] |
| Keratin Source | Processing Parameters | Reference | |
|---|---|---|---|
| Extracting Solution | Conditions | ||
| Wool | KOH; CaO 5% 10% 15% | 140 °C/170 °C/1 h | [88] |
| Wool (red sheep hair) | Extractive liquor: NaOH 0.5 N Fiber to liquid ratio 1:20 | 60 °C; 3 h | [82] |
| Feathers | NaOH 0.75 N; | 60 °C; 45 min Yield 82% | [62] |
| Keratin Source | Processing Parameters | Reference | |
|---|---|---|---|
| Extracting Solution | Conditions | ||
| Feathers | [Choline][thioglycolate] Solid, liquid ratio: 1:2 | 130 °C; 10 h Solubility up to 45% | [94] |
| Feathers | [Bmim]Cl Solid, liquid ratio: 1:2 | 130 °C; 10 h Solubility up to 51% | [94] |
| Feathers | [Amim]Cl Solid, liquid ratio: 1:2 | 130 °C; 10 h Solubility up to 51% | [94] |
| Feathers | [BMIN]Cl + 10% wt Na2SO3 Solid:liquid ratio: 1:20 | 90 °C; 60 min Keratin yields: 75.1% | [96] |
| Wool | [DBNE]DEP Solid, liquid ratio: 8 wt% | 393 K, 3 h Yield: 0.447 g/g | [97] |
| Wool | [DBNM]DMP Solid, liquid ratio: 8 wt% | 393 K, 3.5 h Yield: 0.4 g/g | [97] |
| Feathers | [Bmim]Cl 500 mg in 20 g | 130 °C; 2 h | [98] |
| Feathers | [Bmim]Cl: dimethylsulfoxide mixture 35:65 500 mg in 20 g Ultrasonic treatment: 200 W | 52 min | [98] |
| Feathers | [Bmim]Cl 500 mg in 20 g Ultrasonic treatment: 200 W | 20 min | [98] |
| Microorganism | Substrate | Degradation Condition | Reference | |
|---|---|---|---|---|
| pH | Temperature (°C) | |||
| Bacillaceae | ||||
| Bacillus subtilis | Feathers, human hair | 9 | 50 | [109] |
| Bacillus cereus Wu2 | Feathers | 7 | 30 | [110] |
| Bacillus sp. FPF-1 | Feathers | 5 | 25 | [111] |
| Bacillus pumilus | Bovine hair | 8 | 35 | [112] |
| Brevibacillus brevis US575 | Feathers, hair | 8 | 55 | [113] |
| Gram negative bacteria | ||||
| Meiothermus sp. 140 | Feathers, wool, hair | 8 | 70 | [114] |
| Stenotrophomonas maltophilia BBE11-1 | Feathers, wool | 9 (7–11) | 40–60 | [115] |
| Vibrio sp. kr2 | Feathers | 6–8 | 30 | [34] |
| Actinobacteria | ||||
| Streptomyces gulbargensi | Feathers | 8 (7–9) | 45 (30–60) | [116] |
| Aphanoascus fulvescens Chrysosporium articulatum | Feathers | 7.5 | 28.7 | [117] |
| Fungi | ||||
| Aspergillus niger | Feathers, human hair, sheep’s wool | 5 | - | [118] |
| Purpureocillium lilacinum | Hair | 6 (4–9) | 60 (20–65) | [119] |
| Trichopyton sp. HA-2 | Chicken feathers | 8 | 35 | [120] |
| Trichoderma asperellum, Trichoderma atroviridae | Feathers | 7.5 | 26 | [29] |
| Fusarium sp. 1A | Horse hair | 7.5 | 27 | [121] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perța-Crișan, S.; Ursachi, C.Ș.; Gavrilaș, S.; Oancea, F.; Munteanu, F.-D. Closing the Loop with Keratin-Rich Fibrous Materials. Polymers 2021, 13, 1896. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111896
Perța-Crișan S, Ursachi CȘ, Gavrilaș S, Oancea F, Munteanu F-D. Closing the Loop with Keratin-Rich Fibrous Materials. Polymers. 2021; 13(11):1896. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111896
Chicago/Turabian StylePerța-Crișan, Simona, Claudiu Ștefan Ursachi, Simona Gavrilaș, Florin Oancea, and Florentina-Daniela Munteanu. 2021. "Closing the Loop with Keratin-Rich Fibrous Materials" Polymers 13, no. 11: 1896. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13111896