Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater
Abstract
:1. Introduction
2. Materials and Methods
- A low pH GLDA (pH = 4) where NaOH was added to raise the pH gradually from 4 to 10.
- A high pH GLDA as received from the manufacturer, the pH measured was 13.7, without NaOH addition.
2.1. Concentric Cylinder Experiments (Standard Conditions)
2.2. Pressure Cell Experiments (HPHT)
3. Results and Discussions
3.1. pH Control
3.2. GLDA pH Impact on Viscosity
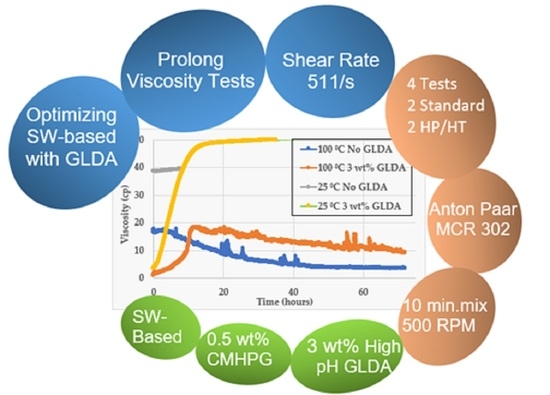
3.3. Prolong Viscosity Tests
3.3.1. Standard Conditions
3.3.2. HPHT Conditions
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Nolte, K.G.; Economides, M.J. Reservoir Stimulation; John Wiley & Sons: Hoboken, NJ, USA, 2000. [Google Scholar]
- Wickard, A.K.; Elmore, R.D.; Heij, G. A Diagenetic Study of the Wolfcamp Shale, Permian Basin, West Texas. In Proceedings of the AAPG Annual Convention and Exhibition, Calgary, AB, Canada, 19–22 June 2016. [Google Scholar]
- Gandossi, L.; Von Estorff, U. An overview of hydraulic fracturing and other formation stimulation technologies for shale gas production. Eur. Commisison Jt. Res. Cent. Technol. 2013, 26347. [Google Scholar] [CrossRef]
- Hoffman, A.; Olsson, G.; Lindström, A. Shale Gas and Hydraulic Fracturing: Framing the Water Issue; SIWI: Stockholm, Sweden, 2014. [Google Scholar]
- Samuel, M.; Polson, D.; Graham, D.; Kordziel, W.; Waite, T.; Waters, G.; Downey, R. Viscoelastic surfactant fracturing fluids: Applications in low permeability reservoirs. In Proceedings of the SPE Rocky Mountain Regional/Low-Permeability Reservoirs Symposium and Exhibition, Denver, CO, USA, 12–15 March 2000. [Google Scholar]
- Das, P.; Rahim, Z. Evaluate Fracturing Fluid Performance for Hydraulic Stimulation in Pre-Khuff Sandstone Reservoirs of Ghawar Gas Field. In Proceedings of the SPE Saudi Arabia Section Technical Symposium and Exhibition, Al-Khobar, Saudi Arabia, 21–24 April 2014. [Google Scholar]
- Montgomery, C. Fracturing fluids. In Proceedings of the ISRM International Conference for Effective and Sustainable Hydraulic Fracturing, Brisbane, Australia, 20–22 May 2013. [Google Scholar]
- Isah, A.; Hiba, M.; Al-Azani, K.; Aljawad, M.S.; Mahmoud, M. A comprehensive review of proppant transport in fractured reservoirs: Experimental, numerical, and field aspects. J. Nat. Gas Sci. Eng. 2021, 88, 103832. [Google Scholar] [CrossRef]
- Fink, J. Petroleum Engineer’s Guide to oil Field Chemicals and Fluids; Gulf Professional Publishing: Oxford, UK, 2021; ISBN 9780128037348. [Google Scholar]
- Barati, R.; Liang, J.-T. A review of fracturing fluid systems used for hydraulic fracturing of oil and gas wells. J. Appl. Polym. Sci. 2014, 131, 1–11. [Google Scholar] [CrossRef]
- Hemmati-Sarapardeh, A.; Varamesh, A.; Husein, M.M.; Karan, K. On the evaluation of the viscosity of nanofluid systems: Modeling and data assessment. Renew. Sustain. Energy Rev. 2018, 81, 313–329. [Google Scholar] [CrossRef]
- Zare, Y.; Park, S.P.; Rhee, K.Y. Analysis of complex viscosity and shear thinning behavior in poly (lactic acid)/poly (ethylene oxide)/carbon nanotubes biosensor based on Carreau–Yasuda model. Results Phys. 2019, 13, 102245. [Google Scholar] [CrossRef]
- Yekeen, N.; Padmanabhan, E.; Idris, A.K. A review of recent advances in foam-based fracturing fluid application in unconventional reservoirs. J. Ind. Eng. Chem. 2018, 66, 45–71. [Google Scholar] [CrossRef]
- Anandan, R.; Johnson, S.; Barati, R. Polyelectrolyte complex stabilized CO2 Foam systems for hydraulic fracturing application. In Proceedings of the SPE Liquids-Rich Basins Conference, Midland, TX, USA, 13–14 September 2017; Volume 2017, pp. 1–19. [Google Scholar]
- Faroughi, S.A.; Pruvot, A.J.-C.J.; McAndrew, J. The rheological behavior of energized fluids and foams with application to hydraulic fracturing: Review. J. Pet. Sci. Eng. 2018, 163, 243–263. [Google Scholar] [CrossRef]
- Harris, P.C. Fracturing-Fluid Additives. J. Pet. Technol. 1988, 40, 1277–1279. [Google Scholar] [CrossRef]
- Mahmoud, M.A.; Abdelgawad, K.Z. Chelating-agent enhanced oil recovery for sandstone and carbonate reservoirs. SPE J. 2015, 20, 483–495. [Google Scholar] [CrossRef]
- Mohammed, M.N.; Mahmoud, M.; Elkatatny, S. Development of a Smart Fracturing Fluid for Tight and Unconventional Reservoirs. In Proceedings of the SPE Annual Technical Conference and Exhibition, San Antonio, TX, USA, 9–11 October 2017. [Google Scholar]
- Gaurina-Međimurec, N.; Brkić, V.; Topolovec, M.; Mijić, P. Fracturing Fluids and Their Application in the Republic of Croatia. Appl. Sci. 2021, 11, 2807. [Google Scholar] [CrossRef]
- Al-Muntasheri, G.A. A Critical Review of Hydraulic Fracturing Fluids over the Last Decade. All Days 2014, 84, 89–114. [Google Scholar] [CrossRef]
- Bahri-Laleh, N.; Hanifpour, A.; Mirmohammadi, S.A.; Poater, A.; Nekoomanesh-Haghighi, M.; Talarico, G.; Cavallo, L. Computational modeling of heterogeneous Ziegler-Natta catalysts for olefins polymerization. Prog. Polym. Sci. 2018, 84, 89–114. [Google Scholar] [CrossRef]
- Abdul Majid, A.B.; Hansen, J.E.; Al-Dahlan, M.N.; Malik, A.R.; Alharbi, M.M.; Al-Suwaigh, M.K. Seawater based fracturing fluid: A game changer in hydraulic fracturing applications in Saudi Arabia. In Proceedings of the 20th Middle East Oil & Gas Show and Conference (MEOS 2017), Manama, Bahrain, 6–9 March 2017. [Google Scholar]
- Alohaly, M.; BinGhanim, A.; Rahal, R.; Rahim, S. Seawater Fracturing Fluid Development Challenges: A Comparison between Seawater-Based and Freshwater-Based Fracturing Fluids Using Two Types of Guar Gum Polymers. In Proceedings of the SPE Kingdom of Saudi Arabia Annual Technical Symposium and Exhibition, Dammam, Saudi Arabia, 24–27 April 2017. [Google Scholar]
- Wilson, A. A Comparison Between Seawater-Based and Freshwater-Based Fracturing Fluids. J. Pet. Technol. 2017, 69, 46–47. [Google Scholar] [CrossRef]
- Szopinski, D.; Kulicke, W.-M.; Luinstra, G.A. Structure–property relationships of carboxymethyl hydroxypropyl guar gum in water and a hyperentanglement parameter. Carbohydr. Polym. 2015, 119, 159–166. [Google Scholar] [CrossRef]
- Hu, Y.Y.; Kishore, T.; Maxey, J.; Loveless, D. Effects of crosslinking chemistry on proppant suspension in guar networks. In Proceedings of the SPE International Symposium on Oilfield Chemistry, The Woodlands, TX, USA, 6–7 December 2015. [Google Scholar]
- Hanifpour, A.; Bahri-Laleh, N.; Nekoomanesh-Haghighi, M.; Poater, A. Coordinative chain transfer polymerization of 1-decene in the presence of a Ti-based diamine bis(phenolate) catalyst: A sustainable approach to produce low viscosity PAOs. Green Chem. 2020, 22, 4617–4626. [Google Scholar] [CrossRef]
- Nishimura, Y.; Chung, J.; Muradyan, H.; Guan, Z. Silyl Ether as a Robust and Thermally Stable Dynamic Covalent Motif for Malleable Polymer Design. J. Am. Chem. Soc. 2017, 139, 14881–14884. [Google Scholar] [CrossRef]
- Zhang, Z.P.; Rong, M.Z.; Zhang, M.Q. Polymer engineering based on reversible covalent chemistry: A promising innovative pathway towards new materials and new functionalities. Prog. Polym. Sci. 2018, 80, 39–93. [Google Scholar] [CrossRef]
- Hassan, A.; Mahmoud, M.; Bageri, B.S.; Aljawad, M.S.; Kamal, M.S.; Barri, A.A.; Hussein, I.A. Applications of Chelating Agents in the Upstream Oil and Gas Industry: A Review. Energy Fuels 2020, 34, 15593–15613. [Google Scholar] [CrossRef]
- Mahmoud, M. Waterless Fracturing (Foam Fracturing). Lecture notes in king Fahd University of Petroleum and Minerals. 2019. [Google Scholar]
- Kamal, M.S.; Mohammed, M.; Mahmoud, M.; Elkatatny, S. Development of Chelating Agent-Based Polymeric Gel System for Hydraulic Fracturing. Energies 2018, 11, 1663. [Google Scholar] [CrossRef] [Green Version]
- Lepage, J.; De Wolf, C.; Nemelaar, J.; Nasr-El-Din, H. An Environmentally Friendly Stimulation Fluid for High-Temperature Applications. SPE J. 2011, 16, 104–110. [Google Scholar] [CrossRef]
- Bretti, C.; Majlesi, K.; De Stefano, C.; Sammartano, S. Thermodynamic Study on the Protonation and Complexation of GLDA with Ca2+ and Mg2+ at Different Ionic Strengths and Ionic Media at 298.15 K. J. Chem. Eng. Data 2016, 61, 1895–1903. [Google Scholar] [CrossRef]
- Elsarawy, A.M.; Nasr-El-Din, H.A.; Cawiezel, K.E. Compatibility and Rheology of High-pH Borate Gels Prepared with Produced Water for Hydraulic-Fracturing Applications. SPE Prod. Oper. 2018, 33, 179–195. [Google Scholar] [CrossRef]
- Prakash, C.; Raykov, T.; Koalsa, B.; Belakshe, R.; Janiczek, P. Hydraulic Fracturing Application of New Seawater-Based Clean Fluid. In Proceedings of the Abu Dhabi International Petroleum Exhibition & Conference, Abu Dhabi, United Arab Emirates, 7–10 November 2016. [Google Scholar]
- Longo, S.; Di Federico, V.; Archetti, R.; Chiapponi, L.; Ciriello, V.; Ungarish, M. On the axisymmetric spreading of non-Newtonian power-law gravity currents of time-dependent volume: An experimental and theoretical investigation fo-cused on the inference of rheological parameters. J. Non-Newton. Fluid Mech. 2013, 201, 69–79. [Google Scholar] [CrossRef]












| Compound (Anhydrite) | g/L |
|---|---|
| NaHCO3 | 0.165 |
| Na2SO4 | 6.339 |
| NaCl | 41.172 |
| CaCl2.2H2O | 2.387 |
| MgCl2.6H2O | 17.644 |
| Total dissolved solids (TDS) | 67.707 |
| Ambient Conditions | HPHT | Comparison |
|---|---|---|
|
|
|
| pH tests | Temperature tests | |
|
|
|
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Othman, A.; Aljawad, M.S.; Mahmoud, M.; Kamal, M.S.; Patil, S.; Bataweel, M. Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater. Polymers 2021, 13, 2111. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132111
Othman A, Aljawad MS, Mahmoud M, Kamal MS, Patil S, Bataweel M. Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater. Polymers. 2021; 13(13):2111. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132111
Chicago/Turabian StyleOthman, Amro, Murtada Saleh Aljawad, Mohamed Mahmoud, Muhammad Shahzad Kamal, Shirish Patil, and Mohammed Bataweel. 2021. "Chelating Agents Usage in Optimization of Fracturing Fluid Rheology Prepared from Seawater" Polymers 13, no. 13: 2111. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132111