Biocomposites of Low-Density Polyethylene Plus Wood Flour or Flax Straw: Biodegradation Kinetics across Three Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Composite Preparation
2.3. Measurements
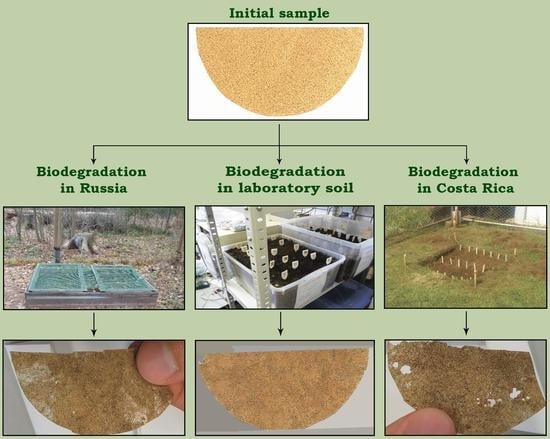
- Prepared soil mix under laboratory conditions (constant temperature (23 ± 3 °C) and constant humidity (60%)).
- Prepared soil mix under ambient field environmental conditions in Moscow region, (Kubinka, Moscow region, Russia). A natural soil layer was removed (depth of 30 cm) and replaced with the prepared soil mix. There was no barrier between natural soil and the prepared soil mix.
- Natural soil (not prepared soil mix) under ambient field environmental conditions in an experimental field at the Universidad Nacional (Heredia, Costa Rica) [30].
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Brebu, M. Environmental Degradation of Plastic Composites with Natural Fillers—A Review. Polymers 2020, 12, 166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Way, C.; Dean, K.; Wu, D.Y.; Palombo, E. Biodegradation of sequentially surface treated lignocellulose reinforced polylactic acid composites: Carbon dioxide evolution and morphology. Polym. Degrad. Stab. 2012, 97, 430–438. [Google Scholar] [CrossRef]
- Thompson, A.A.; Samuelson, M.B.; Kadoma, I.; Soto-Cantu, E.; Drijber, R.; Wortman, S.E. Degradation Rate of Bio-based Agricultural Mulch is Influenced by Mulch Composition and Biostimulant Application. J. Polym. Environ. 2019, 27, 498–509. [Google Scholar] [CrossRef]
- Siracusa, V.; Ingrao, C.; Karpova, S.G.; Olkhov, A.A.; Iordanskii, A.L. Gas transport and characterization of poly(3 hydroxybutyrate) films. Eur. Polym. J. 2017, 91, 149–161. [Google Scholar] [CrossRef]
- Rosa, D.D.S.; Rodrigues, T.C.; Gracas Fassina Guedes, C.D.; Calil, M.R. Effect of thermal aging on the biodegradation of PCL, PHB-V, and their blends with starch in soil compost. J. Appl. Polym. Sci. 2003, 89, 3539–3546. [Google Scholar] [CrossRef]
- Ali, S.F.A. Biodegradation Properties of Poly-ε-caprolactone, Starch and Cellulose Acetate Butyrate Composites. J. Polym. Environ. 2014, 22, 359–364. [Google Scholar] [CrossRef]
- Pantyukhov, P.; Kolesnikova, N.; Popov, A. Preparation, structure, and properties of biocomposites based on low-density polyethylene and lignocellulosic fillers. Polym. Compos. 2016, 37, 1461–1472. [Google Scholar] [CrossRef]
- Zykova, A.K.; Pantyukhov, P.V.; Kolesnikova, N.N.; Monakhova, T.V.; Popov, A.A. Influence of Filler Particle Size on Physical Properties and Biodegradation of Biocomposites Based on Low-Density Polyethylene and Lignocellulosic Fillers. J. Polym. Environ. 2018, 26, 1343–1354. [Google Scholar] [CrossRef]
- Marcovich, N.E.; Villar, M.A. Thermal and mechanical characterization of linear low-density polyethylene/wood flour composites. J. Appl. Polym. Sci. 2003, 90, 2775–2784. [Google Scholar] [CrossRef]
- Xiong, C.; Qi, R.; Wang, Y. Wood-thermoplastic composites from wood flour and high-density polyethylene. J. Appl. Polym. Sci. 2009, 114, 1160–1168. [Google Scholar] [CrossRef]
- Mastalygina, E.; Popov, A. Mechanical Properties and Stress-Strain Behaviour of Binary and Ternary Composites Based on Polyolefins and Vegetable Fillers. In Solid State Phenomena; Trans Tech Publications Ltd.: Stafa-Zurich, Switzerland, 2017; Volume 265, pp. 221–226. [Google Scholar] [CrossRef]
- Mikhaylov, I.A.; Sukhareva, K.V.; Andriasyan, Y.O.; Popov, A.A.; Vorontsov, N.V. Mechanochemical modification of natural Rubber. In Proceedings of the International Conference on Advanced Materials with Hierarchical Structure for New Technologies and Reliable Structures, Tomsk, Russia, 19–23 September 2016; AIP Publishing: Melville, NY, USA, 2016; Volume 1783, p. 020153. [Google Scholar]
- Żelaziński, T.; Ekielski, A.; Tulska, E.; Vladut, V.; Durczak, K. Wood dust application for improvement of selected properties of thermoplastic starch. INMATEH Agric. Eng. 2019, 58, 37–44. [Google Scholar] [CrossRef]
- Jullanun, P.; Yoksan, R. Morphological characteristics and properties of TPS/PLA/cassava pulp biocomposites. Polym. Test. 2020, 88, 106522. [Google Scholar] [CrossRef]
- Dikobe, D.; Luyt, A. Thermal and mechanical properties of PP/HDPE/wood powder and MAPP/HDPE/wood powder polymer blend composites. Thermochim. Acta 2017, 654, 40–50. [Google Scholar] [CrossRef] [Green Version]
- Rambe, A.M. Biodegradation of Low Density Polyethylene (LDPE) Composite Filled with Cellulose and Cellulose Acetate. In Advanced Materials Research; Trans Tech Publications: Stafa-Zurich, Switzerland, 2014; Volume 896, pp. 314–317. [Google Scholar] [CrossRef]
- Kumar, R.; Yakubu, M.K.; Anandjiwala, R.D. Biodegradation of flax fiber reinforced poly lactic acid. Express Polym. Lett. 2010, 4, 423–430. [Google Scholar] [CrossRef]
- Chattopadhyay, S.K.; Singh, S.; Pramanik, N.; Niyogi, U.K.; Khandal, R.K.; Uppaluri, R.; Ghoshal, A.K. Biodegradability studies on natural fibers reinforced polypropylene composites. J. Appl. Polym. Sci. 2011, 121, 2226–2232. [Google Scholar] [CrossRef]
- Mastalygina, E.; Pantyukhov, P.; Popov, A. Biodegradation of natural reinforcing fillers for polymer composites. IOP Conf. Ser. Mater. Sci. Eng. 2018, 369, 012044. [Google Scholar] [CrossRef]
- Lam, E.U.; Pliego, M.G.G.; Pérez, V.G.M.; Ramirez, A.M. Determination of Mechanical Properties of Biodegradable Composites Made by Pine Resin Corn Fibers and Henequen Fibers. Key Eng. Mater. 2012, 517, 422–429. [Google Scholar] [CrossRef]
- Majeed, K.; Hassan, A.; Abu Bakar, A. Barrier, Biodegradation, and mechanical properties of (Rice husk)/(Montmorillonite) hybrid filler-filled low-density polyethylene nanocomposite films. J. Vinyl Addit. Technol. 2015, 23, 162–171. [Google Scholar] [CrossRef]
- Karamanlioglu, M.; Robson, G.D. The influence of biotic and abiotic factors on the rate of degradation of poly(lactic) acid (PLA) coupons buried in compost and soil. Polym. Degrad. Stab. 2013, 98, 2063–2071. [Google Scholar] [CrossRef]
- Li, C.; Moore-Kucera, J.; Miles, C.; Leonas, K.; Lee, J.; Corbin, A.; Inglis, D. Degradation of Potentially Biodegradable Plastic Mulch Films at Three Diverse U.S. Locations. Agroecol. Sustain. Food Syst. 2014, 38, 861–889. [Google Scholar] [CrossRef]
- Mohamad, N.; Latiff, A.A.; Maulod, H.E.A.; Azam, M.A.; Manaf, M.E.A. A Sustainable Polymer Composite from Recycled Polypropylene Filled with Shrimp Shell Waste. Polym. Technol. Eng. 2014, 53, 167–172. [Google Scholar] [CrossRef]
- Brodhagen, M.; Peyron, M.; Miles, C.; Inglis, D.A. Biodegradable plastic agricultural mulches and key features of microbial degradation. Appl. Microbiol. Biotechnol. 2014, 99, 1039–1056. [Google Scholar] [CrossRef] [PubMed]
- Dharmalingam, S.; Hayes, D.G.; Wadsworth, L.C.; Dunlap, R.N.; Debruyn, J.M.; Lee, J.; Wszelaki, A.L. Soil Degradation of Polylactic Acid/Polyhydroxyalkanoate-Based Nonwoven Mulches. J. Polym. Environ. 2015, 23, 302–315. [Google Scholar] [CrossRef]
- Shabani, F.; Kumar, L.; Esmaeili, A. A modelling implementation of climate change on biodegradation of Low-Density Polyethylene (LDPE) by Aspergillus niger in soil. Glob. Ecol. Conserv. 2015, 4, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Jakubowicz, I.; Yarahmadi, N.; Petersen, H. Evaluation of the rate of abiotic degradation of biodegradable polyethylene in various environments. Polym. Degrad. Stab. 2006, 91, 1556–1562. [Google Scholar] [CrossRef]
- Hoshino, A.; Sawada, H.; Yokota, M.; Tsuji, M.; Fukuda, K.; Kimura, M. Influence of weather conditions and soil properties on degradation of biodegradable plastics in soil. Soil Sci. Plant. Nutr. 2001, 47, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Zykova, A.; Pantyukhov, P.; Morokov, E.; Ramos, C.C.; Nikolaeva, S.; Popov, A. Influence of different climate conditions on biodegradation kinetics of biocomposites based on low-density polyethylene and vegetable fillers. In Proceedings of the 9th International Conference on “Times of Polymers and Composites”: From Aerospace to Nanotechnology, Ischia, Italy, 17–21 June 2018; AIP Publishing: Melville, NY, USA, 2018; Volume 1981, p. 020019. [Google Scholar] [CrossRef]
- Strömberg, E.; Karlsson, S. The effect of biodegradation on surface and bulk property changes of polypropylene, recycled polypropylene and polylactide biocomposites. Int. Biodeterior. Biodegrad. 2009, 63, 1045–1053. [Google Scholar] [CrossRef]
- Borsoi, C.; Berwig, K.H.; Scienza, L.C.; Zattera, A.J. The photodegradation and biodegradation of rEPS/curaua fiber composites. Polym. Compos. 2013, 34, 967–977. [Google Scholar] [CrossRef]
- Nair, K.C.M.; Thomas, S. Effect of interface modification on the mechanical properties of polystyrene-sisal fiber composites. Polym. Compos. 2003, 24, 332–343. [Google Scholar] [CrossRef]
- Mastalygina, E.E.; Popov, A.; Pantyukhov, P.V. Effect of biobased fillers nature on biodeterioration of hybrid polyethylene composites by mold fungi. IOP Conf. Ser. Mater. Sci. Eng. 2017, 213, 012011. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-S.; Kim, H.-J.; Lee, J.-W.; Choi, I.-G. Biodegradability of bio-flour filled biodegradable poly(butylene succinate) bio-composites in natural and compost soil. Polym. Degrad. Stab. 2006, 91, 1117–1127. [Google Scholar] [CrossRef]
- Barton-Pudlik, J.; Czaja, K.; Grzymek, M.; Lipok, J. Evaluation of wood-polyethylene composites biodegradability caused by filamentous fungi. Int. Biodeterior. Biodegrad. 2017, 118, 10–18. [Google Scholar] [CrossRef]
- Fabiyi, J.S.; McDonald, A.G.; Morrell, J.J.; Freitag, C. Effects of wood species on durability and chemical changes of fungal decayed wood plastic composites. Compos. Part A Appl. Sci. Manuf. 2011, 42, 501–510. [Google Scholar] [CrossRef]
- Catto, A.L.; Montagna, L.S.; Santana, R.M. Abiotic and biotic degradation of post-consumer polypropylene/ethylene vinyl acetate: Wood flour composites exposed to natural weathering. Polym. Compos. 2015, 38, 571–582. [Google Scholar] [CrossRef]
- Sheik, S.; Chandrashekar, K.; Swaroop, K.; Somashekarappa, H. Biodegradation of gamma irradiated low density polyethylene and polypropylene by endophytic fungi. Int. Biodeterior. Biodegrad. 2015, 105, 21–29. [Google Scholar] [CrossRef]
- Degli-Innocenti, F. Biodegradability and Compostability. In Biodegradable Polymers and Plastics; Chiellini, E., Solaro, R., Eds.; Springer Science and Business Media LLC: Berlin/Heidelberg, Germany, 2003; pp. 33–45. [Google Scholar]
- Zykova, A.; Pantyukhov, P.; Popov, A. Ethylene-octene copolymer-wood flour/oil flax straw biocomposites: Effect of filler type and content on mechanical properties. Polym. Eng. Sci. 2017, 57, 756–763. [Google Scholar] [CrossRef]
- Poletto, M.; Zeni, M.; Zattera, A.J. Dynamic mechanical analysis of recycled polyst:10yrene composites reinforced with wood flour. J. Appl. Polym. Sci. 2011, 125, 935–942. [Google Scholar] [CrossRef]
- Zykova, A.K.; Pantyukhov, P.; Monakhova, T.V.; Shatalova, O.V.; Krivandin, A.V.; Popov, A.A. Thermal oxidation kinetics and its effect on properties and structure of LDPE films. In Proceedings of the International Conference on Advanced Materials with Hierarchical Structure for New Technologies and Reliable Structures, Tomsk, Russia, 1–5 October 2019; AIP Publishing: Melville, NY, USA, 2019; Volume 2167, p. 020412. [Google Scholar] [CrossRef]
- Bonhomme, S.; Cuer, A.; Delort, A.-M.; Lemaire, J.; Sancelme, M.; Scott, G. Environmental biodegradation of polyethylene. Polym. Degrad. Stab. 2003, 81, 441–452. [Google Scholar] [CrossRef]
- Gumargalieva, K.; Kalinina, I.; Mironova, S.; Zaikov, G. Biodegradation of polymers and adhesion properties of microorganism cells. Polym. Degrad. Stab. 1995, 47, 363–368. [Google Scholar] [CrossRef]








| Soil Property | Russian Soil Mix | Costa Rica Field Soil |
|---|---|---|
| Organic matter (g kg−1) | 138 | - |
| Organic carbon (g kg−1) | - | 33.4 |
| pH (1:1 soil:water) | 6.5 | 6.2 |
| Organic nitrogen (g kg−1) | - | 2.8 |
| Nitrate (mg kg−1) | 728 | 1.6 |
| Ammonium (mg kg−1) | 21 | 3.6 |
| Phosphorus (mg kg−1) | 421 | 8 |
| Potassium (mg kg−1) | 856 | 417 |
| Climatic Conditions | Time of Exposition, Months | Weight Loss, % | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 70 LDPE/30 WF | 70 LDPE/30 FS | ||||||||
| 0–80 | 80–140 | 140–200 | 0–200 | 0–80 | 80–140 | 140–200 | 0–200 | ||
| Laboratory conditions | 0.5 | 0 | 0.2 | 0.8 | 1.3 | 4.8 | 7.2 | 8.61 | 6.2 |
| 2 | 0.7 | 2.8 | 6.7 | 8.6 | 14.2 | 16.6 | 17.60 | 14.8 | |
| 4 | 4.2 | 8.0 | 12.2 | 12.9 | 16.6 | 18.8 | 19.94 | 17.4 | |
| 6 | 7.7 | 11.0 | 14.7 | 15.5 | 17.8 | 19.9 | 21.20 | 18.5 | |
| 8 | 11.4 | 13.6 | 16.6 | 17.6 | 18.8 | 20.8 | 22.04 | 19.4 | |
| 10 | 13.1 | 14.6 | 17.3 | 18.7 | 18.8 | 21.0 | 22.38 | 19.8 | |
| 12 | 13.3 | 14.9 | 17.5 | 19.0 | 18.8 | 20.9 | 22.38 | 19.3 | |
| 14 | 15.2 | 16.3 | 18.2 | 20.1 | 19.2 | 21.5 | 23.05 | 20.1 | |
| 16 | 15.5 | 16.5 | 18.7 | 20.4 | 19.4 | 21.5 | 23.14 | 20.2 | |
| 20 | 16.0 | 16.9 | 19.2 | 21.1 | 19.7 | 21.7 | 23.30 | 20.7 | |
| Open air, Russia | 6 | 6.2 | 7.2 | 8.2 | 9.2 | 10.2 | 11.2 | 12.2 | 13.2 |
| 10 | 6.5 | 7.5 | 8.5 | 9.5 | 10.5 | 11.5 | 12.5 | 13.5 | |
| 20 | 13.2 | 15.4 | 17.2 | 19.5 | 19.3 | 20.7 | 22.0 | 20.1 | |
| Open air, Costa Rica | 2 | 5.2 | 6.1 | 8.4 | 21.9 | 17.5 | 18.9 | 21.3 | 17.1 |
| 6 | 10.4 | 12.2 | 15.2 | 26.7 | 19.3 | 21.4 | 24.9 | 20.7 | |
| 12 | 28.4 * | 18.1 | 20.5 | 30.5 | 22.1 | 24.7 | 28.6 | 24.9 | |
| 20 | 30.9 * | 20.8 | 23.5 | 31.8 | 23.5 | 26.1 | 30.6 | 26.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zykova, A.K.; Pantyukhov, P.V.; Mastalygina, E.E.; Chaverri-Ramos, C.; Nikolaeva, S.G.; Saavedra-Arias, J.J.; Popov, A.A.; Wortman, S.E.; Poletto, M. Biocomposites of Low-Density Polyethylene Plus Wood Flour or Flax Straw: Biodegradation Kinetics across Three Environments. Polymers 2021, 13, 2138. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132138
Zykova AK, Pantyukhov PV, Mastalygina EE, Chaverri-Ramos C, Nikolaeva SG, Saavedra-Arias JJ, Popov AA, Wortman SE, Poletto M. Biocomposites of Low-Density Polyethylene Plus Wood Flour or Flax Straw: Biodegradation Kinetics across Three Environments. Polymers. 2021; 13(13):2138. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132138
Chicago/Turabian StyleZykova, Anna K., Petr V. Pantyukhov, Elena E. Mastalygina, Christian Chaverri-Ramos, Svetlana G. Nikolaeva, Jose J. Saavedra-Arias, Anatoly A. Popov, Sam E. Wortman, and Matheus Poletto. 2021. "Biocomposites of Low-Density Polyethylene Plus Wood Flour or Flax Straw: Biodegradation Kinetics across Three Environments" Polymers 13, no. 13: 2138. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132138