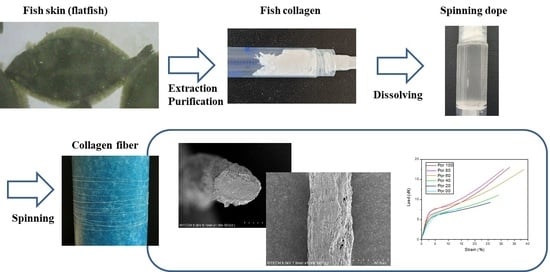
Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Material Analysis
2.3. Fiber Spinning
2.4. Mechanical Analysis
3. Results and Discussions
3.1. Material Analysis
3.2. Structure and Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ferreira, A.M.; Gentile, P.; Chiono, V.; Ciardelli, G. Collagen for bone tissue regeneration. Acta Biomater. 2012, 8, 3191–3200. [Google Scholar] [CrossRef]
- Meyer, M. Processing of collagen based biomaterials and the resulting materials properties. Biomed. Eng. Online 2019, 18, 24. [Google Scholar] [CrossRef] [Green Version]
- Norris, W.D.; Steele, J.G.; Johnson, G.; Underwood, P.A. Serum enhancement of human endothelial cell attachment to and spreading on collagens I and IV does not require serum fibronectin or vitronectin. J. Cell Sci. 1990, 95, 255. [Google Scholar] [CrossRef]
- El-Rashidy, A.A.; Gad, A.; Abu-Hussein, A.E.-H.G.; Habib, S.I.; Badr, N.A.; Hashem, A.A. Chemical and biological evaluation of Egyptian Nile Tilapia (Oreochromis niloticas) fish scale collagen. Int. J. Biol. Macromol. 2015, 79, 618–626. [Google Scholar] [CrossRef]
- Gautieri, A.; Vesentini, S.; Redaelli, A.; Buehler, M.J. Hierarchical Structure and Nanomechanics of Collagen Microfibrils from the Atomistic Scale Up. Nano Lett. 2011, 11, 757–766. [Google Scholar] [CrossRef]
- Burjanadze, T.V. Hydroxyproline content and location in relation to collagen thermal stability. Biopolymers 1979, 18, 931–938. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, M.; Benjakul, S. Extraction and characterisation of pepsin-solubilised collagen from the skin of unicorn leatherjacket (Aluterus monocerous). Food Chem. 2010, 120, 817–824. [Google Scholar] [CrossRef]
- Elango, J.; Zhang, J.; Bao, B.; Palaniyandi, K.; Wang, S.; Wenhui, W.; Robinson, J.S. Rheological, biocompatibility and osteogenesis assessment of fish collagen scaffold for bone tissue engineering. Int. J. Biol. Macromol. 2016, 91, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Brett, D. A Review of Collagen and Collagen-based Wound Dressings. Wounds 2008, 20, 347–356. [Google Scholar] [PubMed]
- Subhan, F.; Hussain, Z.; Tauseef, I.; Shehzad, A.; Wahid, F. A review on recent advances and applications of fish collagen. Crit. Rev. Food Sci. Nutr. 2021, 61, 1027–1037. [Google Scholar] [CrossRef] [PubMed]
- Bao, Z.; Sun, Y.; Rai, K.; Peng, X.; Wang, S.; Nian, R.; Xian, M. The promising indicators of the thermal and mechanical properties of collagen from bass and tilapia: Synergistic effects of hydroxyproline and cysteine. Biomater. Sci. 2018, 6, 3042–3052. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Mielcarek, S.; Włodarczyk, D. Comparison of thermal properties of fish collagen and bovine collagen in the temperature range 298–670 K. Mater. Sci. Eng. C 2017, 80, 468–471. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Fitié, C.F.C.; van der Werf, K.O.; Bennink, M.L.; Dijkstra, P.J.; Feijen, J. Mechanical properties of single electrospun collagen type I fibers. Biomaterials 2008, 29, 955–962. [Google Scholar] [CrossRef] [PubMed]
- Zeugolis, D.I.; Khew, S.T.; Yew, E.S.; Ekaputra, A.K.; Tong, Y.W.; Yung, L.Y.; Hutmacher, D.W.; Sheppard, C.; Raghunath, M. Electro-spinning of pure collagen nano-fibres-just an expensive way to make gelatin? Biomaterials 2008, 29, 2293–2305. [Google Scholar] [CrossRef]
- Tronci, G.; Kanuparti, R.S.; Arafat, M.T.; Yin, J.; Wood, D.J.; Russell, S.J. Wet-spinnability and crosslinked fibre properties of two collagen polypeptides with varied molecular weight. Int. J. Biol. Macromol. 2015, 81, 112–120. [Google Scholar] [CrossRef] [Green Version]
- Liu, H.; Li, D.; Guo, S. Studies on collagen from the skin of channel catfish (Ictalurus punctaus). Food Chem. 2007, 101, 621–625. [Google Scholar] [CrossRef]
- Zeng, S.-K.; Zhang, C.-H.; Lin, H.; Yang, P.; Hong, P.-Z.; Jiang, Z. Isolation and characterisation of acid-solubilised collagen from the skin of Nile tilapia (Oreochromis niloticus). Food Chem. 2009, 116, 879–883. [Google Scholar] [CrossRef]
- Li, J.; Wang, M.; Qiao, Y.; Tian, Y.; Liu, J.; Qin, S.; Wu, W. Extraction and characterization of type I collagen from skin of tilapia (Oreochromis niloticus) and its potential application in biomedical scaffold material for tissue engineering. Process Biochem. 2018, 74, 156–163. [Google Scholar] [CrossRef]
- Pati, F.; Adhikari, B.; Dhara, S. Isolation and characterization of fish scale collagen of higher thermal stability. Bioresour. Technol. 2010, 101, 3737–3742. [Google Scholar] [CrossRef] [PubMed]
- Flandin, F.; Buffevant, C.; Herbage, D. A differential scanning calorimetry analysis of the age-related changes in the thermal stability of rat skin collagen. Biochim. Biophys. Acta Protein Struct. Mol. Enzymol. 1984, 791, 205–211. [Google Scholar] [CrossRef]
- Ghanaeian, A.; Soheilifard, R. Mechanical elasticity of proline-rich and hydroxyproline-rich collagen-like triple-helices studied using steered molecular dynamics. J. Mech. Behav. Biomed. Mater. 2018, 86, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Blanco, M.; Vázquez, J.A.; Pérez-Martín, R.I.; Sotelo, C.G. Hydrolysates of Fish Skin Collagen: An Opportunity for Valorizing Fish Industry Byproducts. Mar Drugs 2017, 15. [Google Scholar]
- Tonndorf, R.; Aibibu, D.; Cherif, C. Collagen multifilament spinning. Mater. Sci. Eng. C 2020, 106, 110105. [Google Scholar] [CrossRef] [PubMed]
- Gauza-Włodarczyk, M.; Kubisz, L.; Włodarczyk, D. Amino acid composition in determination of collagen origin and assessment of physical factors effects. Int. J. Biol. Macromol. 2017, 104, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Heu, M.S.; Lee, J.H.; Kim, H.J.; Jee, S.J.; Lee, J.S.; Jeon, Y.-J.; Shahidi, F.; Kim, J.-S. Characterization of acid- and pepsin-soluble collagens from flatfish skin. Food Sci. Biotechnol. 2010, 19, 27–33. [Google Scholar] [CrossRef]
- Sánchez-Tuesta, L.; Reátegui-Pinedo, N.; Salirrosas, D.; Morocho-Jácome, A.L.; Sarruf, F.D.; Martinez, R.M.; Quevedo-León, R.; Fiestas, R.; Ayala-Jara, C.; Baby, A.R.; et al. Preliminary safety evaluation of n-butanol from the collagen extraction process and of collagen extract from Oreochromisniloticus (tilapia) skin oriented for dermocosmetics. Biomed. Pharm. Res. 2021, 18, 1–16. [Google Scholar]
- Matsumoto, R.; Uemura, T.; Xu, Z.; Yamaguchi, I.; Ikoma, T.; Tanaka, J. Rapid oriented fibril formation of fish scale collagen facilitates early osteoblastic differentiation of human mesenchymal stem cells. J. Biomed. Mater. Res. Part A 2015, 103, 2531–2539. [Google Scholar] [CrossRef]
- Armenta, J.M.; Cortes, D.F.; Pisciotta, J.M.; Shuman, J.L.; Blakeslee, K.; Rasoloson, D.; Ogunbiyi, O.; Sullivan, D.J., Jr.; Shulaev, V. Sensitive and rapid method for amino acid quantitation in malaria biological samples using AccQ.Tag ultra performance liquid chromatography-electrospray ionization-MS/MS with multiple reaction monitoring. Anal. Chem. 2010, 82, 548–558. [Google Scholar] [CrossRef] [Green Version]
- Samouillan, V.; Dandurand, J.; Lacabanne, C.; Thoma, R.J.; Adams, A.; Moore, M. Comparison of chemical treatments on the chain dynamics and thermal stability of bovine pericardium collagen. J. Biomed. Mater. Res. A 2003, 64, 330–338. [Google Scholar] [CrossRef]
- Bazrafshan, Z.; Stylios, G.K. Spinnability of collagen as a biomimetic material: A review. Int. J. Biol. Macromol. 2019, 129, 693–705. [Google Scholar] [CrossRef] [PubMed]








| Amino Acid | Porcine | Tilapia | Flatfish |
|---|---|---|---|
| Gly | 35.3 ± 0.3 b | 34.5 ± 0.2 b | 36.8 ± 0.5 a |
| Hyp | 13.3 ± 0.3 a | 12.7 ± 0.2 a | 10.4 ± 1.0 b |
| Pro | 12.8 ± 0.0 a | 12.0 ± 0.1 b | 11.6 ± 0.2 b |
| Ala | 11.1 ± 0.1 b | 11.8 ± 0.1 b | 13.9 ± 0.4 a |
| Arg | 7.9 ± 0.0 b | 7.2 ± 0.1 c | 8.2 ± 0.1 a |
| His | 3.8 ± 0.2 a | 4.1 ± 0.0 a | 2.1 ± 0.0 b |
| Ser | 2.7 ± 0.2 a | 2.7 ± 0.0 a | 2.0 ± 0.0 b |
| Tyr | 2.7 ± 0.2 b | 2.1 ± 0.0 c | 3.1 ± 0.1 a |
| Glu | 2.1 ± 0.2 b | 3.1 ± 0.1 a | 1.4 ± 0.1 c |
| Leu | 2.1 ± 0.1 a | 2.1 ± 0.1 a | 1.9 ± 0.1 a |
| Lys | 2.0 ± 0.0 a | 1.6 ± 0.0 c | 1.8 ± 0.0 b |
| Thr | 1.3 ± 0.0 c | 2.2 ± 0.0 a | 2.1 ± 0.0 b |
| Phe | 1.0 ± 0.0 b | 1.0 ± 0.1 ab | 1.2 ± 0.1 a |
| Asp | 0.7 ± 0.0 b | 0.9 ± 0.0 a | 0.9 ± 0.0 a |
| Ile | 0.7 ± 0.0 a | 0.8 ± 0.0 a | 0.6 ± 0.0 a |
| Cys | 0.3 ± 0.4 a | 0.8 ± 0.0 a | 0.6 ± 0.0 a |
| Met | 0.2 ± 0.2 b | 0.4 ± 0.0 b | 1.2 ± 0.1 a |
| Val | 0.0 ± 0.0 b | 0.1 ± 0.0 a | 0.0 ± 0.0 b |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahn, H.; Gong, D.J.; Lee, H.H.; Seo, J.Y.; Song, K.-M.; Eom, S.J.; Yeo, S.Y. Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers. Polymers 2021, 13, 2151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132151
Ahn H, Gong DJ, Lee HH, Seo JY, Song K-M, Eom SJ, Yeo SY. Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers. Polymers. 2021; 13(13):2151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132151
Chicago/Turabian StyleAhn, Hyunchul, Da Jeong Gong, Hyun Ho Lee, Joo Yeon Seo, Kyung-Mo Song, Su Jin Eom, and Sang Young Yeo. 2021. "Mechanical Properties of Porcine and Fish Skin-Based Collagen and Conjugated Collagen Fibers" Polymers 13, no. 13: 2151. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132151