Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of Thermo-Responsive Cellulose Hydrogel
2.3. Characterization of Thermo-Responsive Cellulose Hydrogel
2.3.1. Sol-Gel Transition Temperature (SGTT)
2.3.2. Functional Group
2.3.3. Surface Morphology and Structure
2.3.4. Rheological Property
2.4. Performance of Thermo-Responsive Cellulose Hydrogel
2.4.1. Swelling and Degradation
2.4.2. In-Vitro Drug Delivery Study
2.4.3. Kinetic Study of Drug Release
2.4.4. Mechanism of Drug Release
2.5. Statistical Analysis
3. Results and Discussion
3.1. Characterization of Thermo-Responsive Cellulose Hydrogel
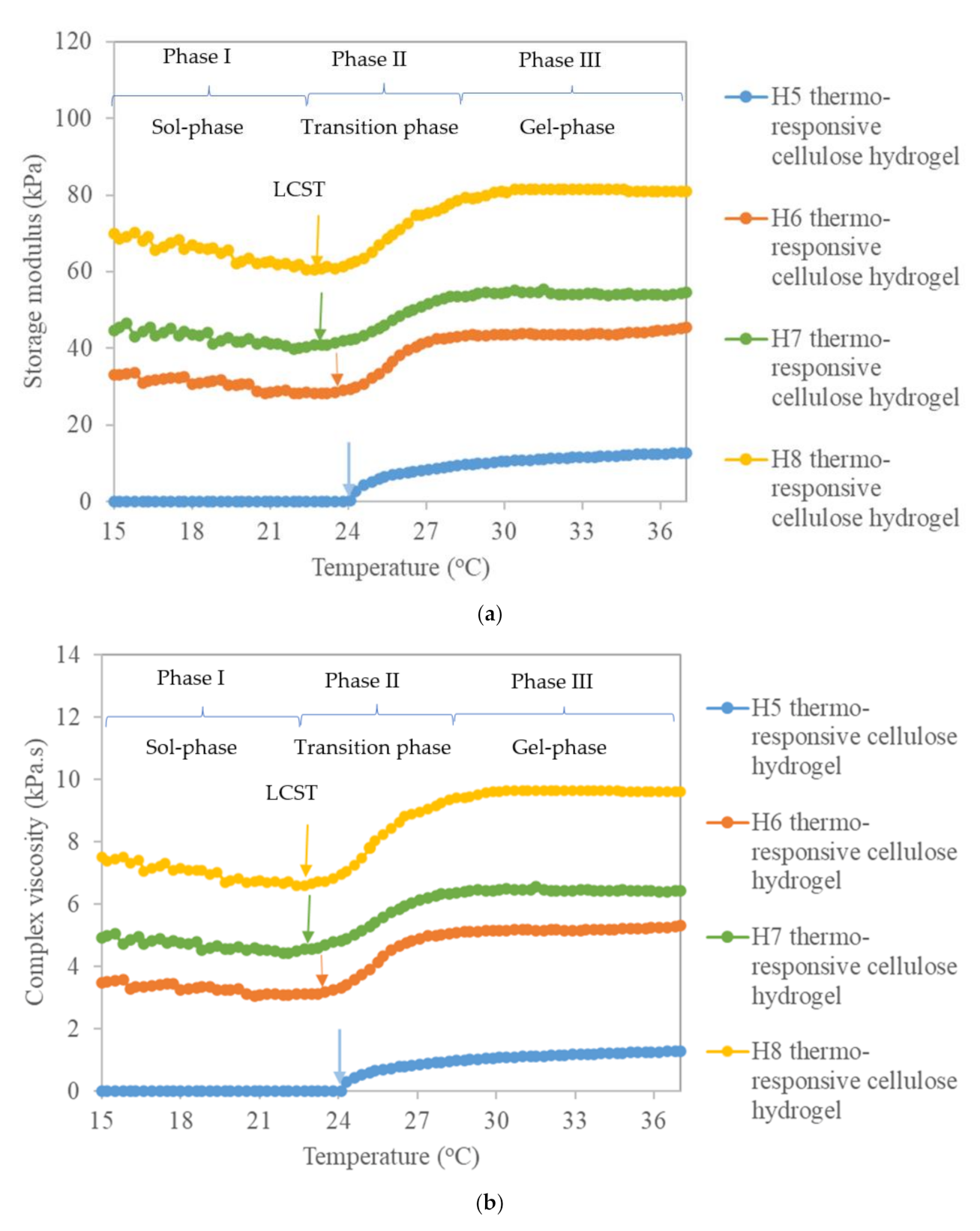
3.1.1. Sol-Gel Transition Temperature (SGTT)
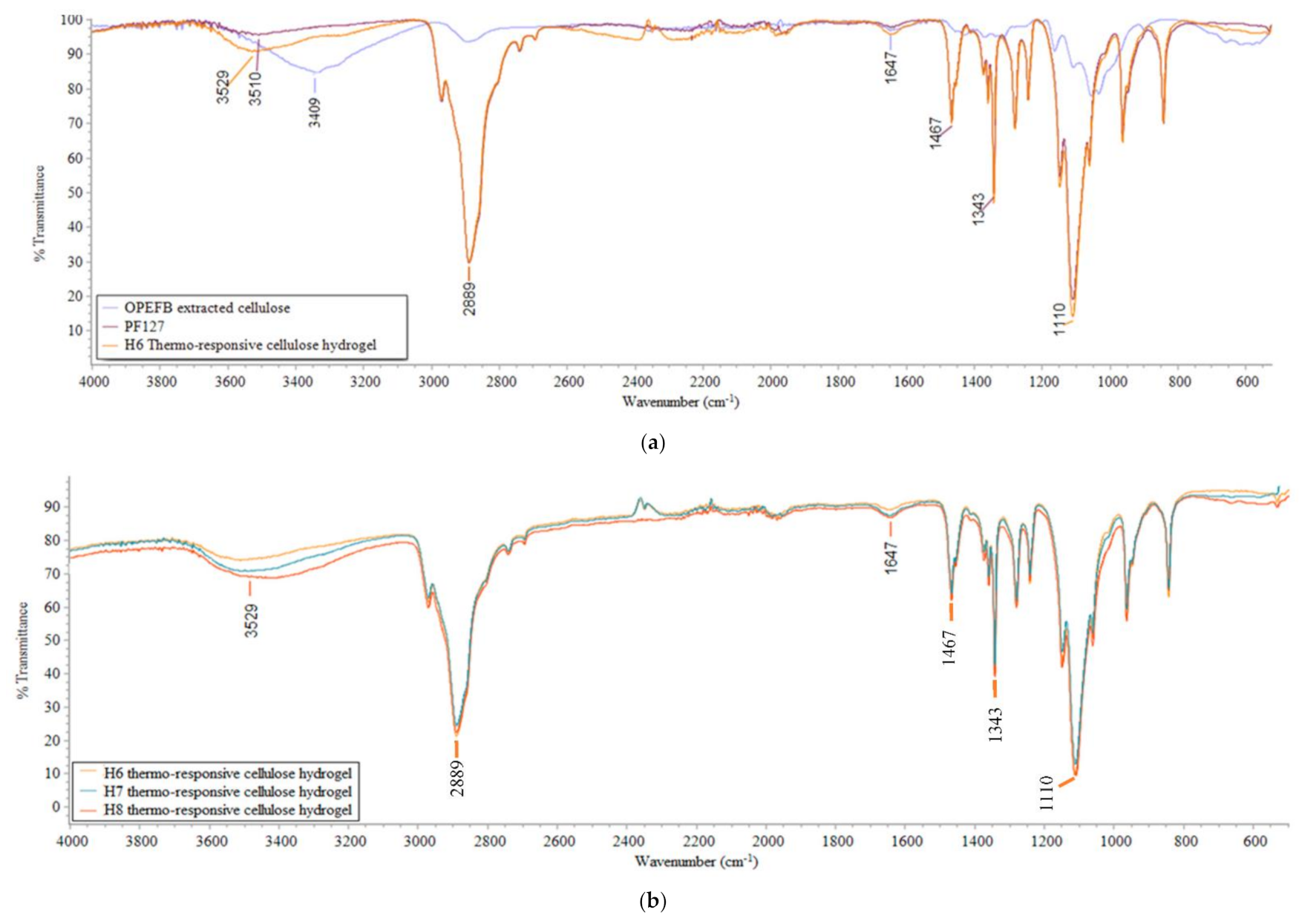
3.1.2. Functional Groups
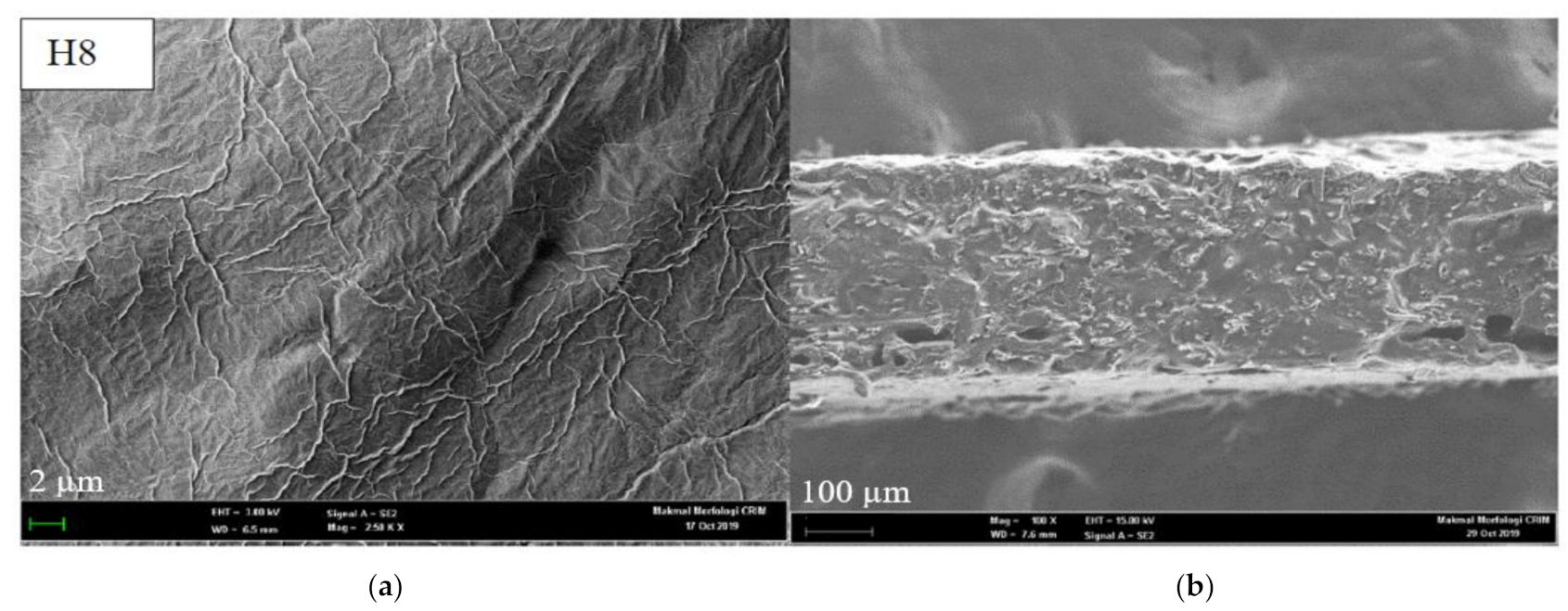
3.1.3. Surface Morphology and Cross-Section View
3.1.4. Rheological Property
3.2. Performance of Thermo-Responsive Cellulose Hydrogel
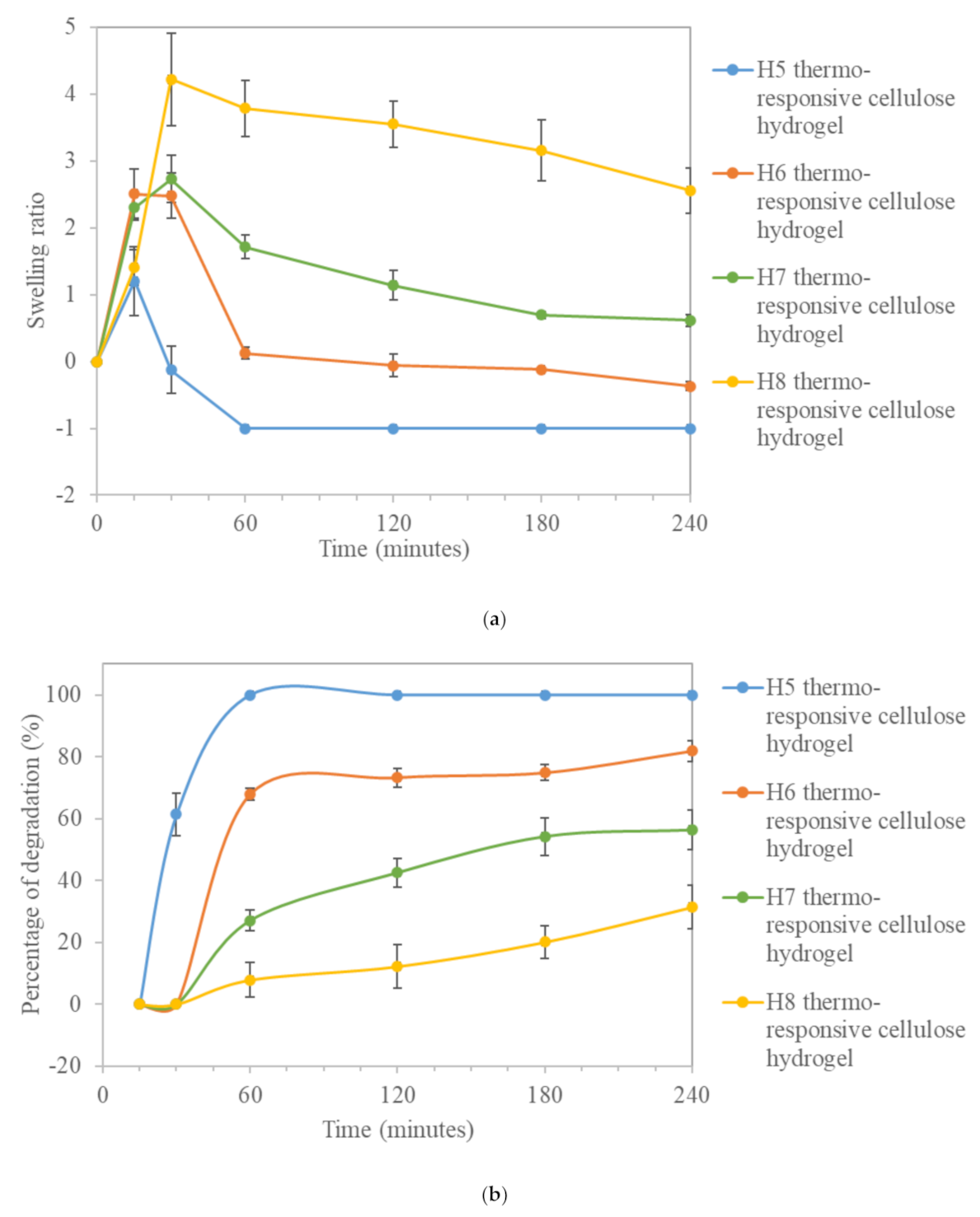
3.2.1. Swelling and Degradation
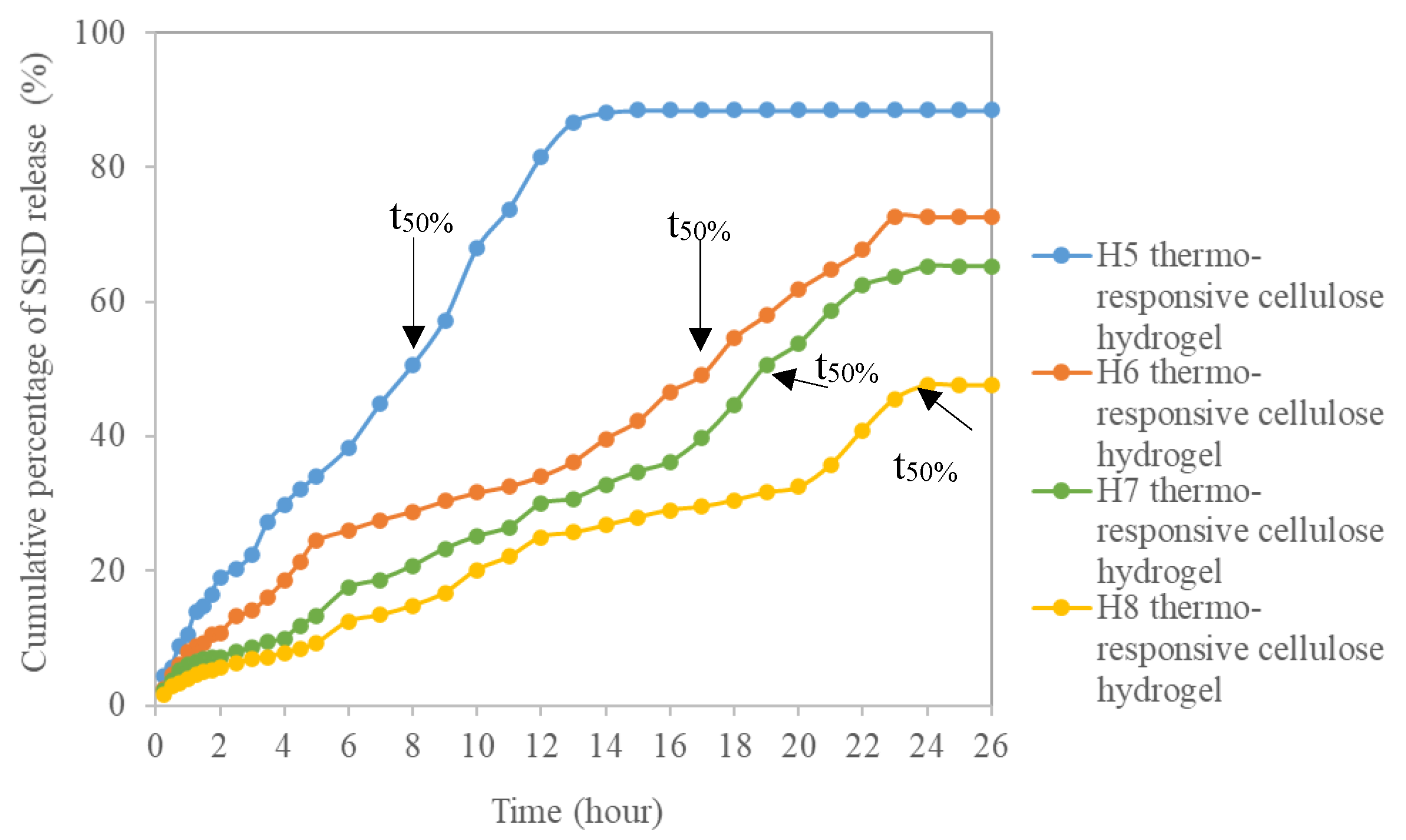
3.2.2. In-Vitro Drug Delivery Study
3.2.3. Kinetic and Mechanism Study of Drug Release
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ribeiro, A.M.; Magalhães, M.; Veiga, F.; Figueiras, A. Cellulose-based hydrogels in topical drug delivery: A challenge in medical devices. In Cellulose-Based Superabsorbent Hydrogels; Springer: Berlin, Germany, 2018; pp. 1–29. [Google Scholar] [CrossRef]
- Boateng, J.S.; Matthews, K.H.; Stevens, H.N.E.; Eccleston, G.M. Wound healing dressings and drug delivery systems: A review. J. Pharm. Sci. 2008, 97, 2892–2923. [Google Scholar] [CrossRef]
- Kathe, K.; Kathpalia, H. Film forming systems for topical and transdermal drug delivery. Asian J. Pharm. Sci. 2017, 12, 487–497. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.; Raw, A.; Lionberger, R.; Yu, L. Generic development of topical dermatologic products: Formulation development, process development, and testing of topical dermatologic products. AAPS J. 2013, 15, 41–52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parsa, M.; Trybała, A.; Malik, D.; Starov, V. Foam in pharmaceutical and medical applications. Curr. Opin. Colloid Interface Sci. 2019, 44, 153–167. [Google Scholar] [CrossRef]
- Escobar-chávez, J.J.; Rodríguez-cruz, I.M.; Domínguez-delgado, C.L.; Díaz-torres, R.; Revilla-vázquez, A.L.; Aléncaster, N.C. Nanocarrier systems for transdermal drug delivery. In Recent Advances in Novel Drug Carrier Systems; IntechOpen: London, UK, 2012; pp. 201–239. [Google Scholar] [CrossRef] [Green Version]
- Chai, Q.; Jiao, Y.; Yu, X. Hydrogels for biomedical applications: Their characteristics and the mechanisms behind them. Gels 2017, 3, 6. [Google Scholar] [CrossRef] [Green Version]
- Ilochonwu, B.C.; Urtti, A.; Hennink, W.E.; Vermonden, T. Intravitreal hydrogels for sustained release of therapeutic proteins. J. Control. Release 2020, 326, 419–441. [Google Scholar] [CrossRef] [PubMed]
- Zubik, K.; Singhsa, P.; Wang, Y.; Manuspiya, H.; Narain, R. Thermo-responsive poly(n-isopropylacrylamide)-cellulose nanocrystals hybrid hydrogels for wound dressing. Polymers 2017, 9, 119. [Google Scholar] [CrossRef] [Green Version]
- Mamidi, N.; Villela Castrejón, J.; González-Ortiz, A. Rational design and engineering of carbon nano-onions reinforced natural protein nanocomposite hydrogels for biomedical applications. J. Mech. Behav. Biomed. Mater. 2020, 104. [Google Scholar] [CrossRef]
- Park, S.H.; Shin, H.S.; Park, S.N. A novel pH-responsive hydrogel based on carboxymethyl cellulose/2-hydroxyethyl acrylate for transdermal delivery of naringenin. Carbohydr. Polym. 2018, 200, 341–352. [Google Scholar] [CrossRef]
- Chang, C.; He, M.; Zhou, J.; Zhang, L. Swelling behaviors of pH- and salt-responsive cellulose-based hydrogels. Macromolecules 2011, 44, 1642–1648. [Google Scholar] [CrossRef]
- Biyani, M.V.; Foster, E.J.; Weder, C. Light-healable supramolecular nanocomposites based on modified cellulose nanocrystals. ACS Macro Lett. 2013, 2, 236–240. [Google Scholar] [CrossRef]
- Reddy, N.N.; Mohan, Y.M.; Varaprasad, K.; Ravindra, S.; Joy, P.A.; Raju, K.M. Magnetic and electric responsive hydrogel–magnetic nanocomposites for drug-delivery application. J. Appl. Polym. Sci. 2010, 122, 1364–1375. [Google Scholar] [CrossRef]
- Kim, Y.; Matsunaga, Y.T. Thermo-responsive polymers and their application as smart biomaterials. J. Mater. Chem. B 2017, 5, 4307–4321. [Google Scholar] [CrossRef] [PubMed]
- Sponchioni, M.; Capasso, U.; Moscatelli, D. Thermo-responsive polymers: Applications of smart materials in drug delivery and tissue engineering. Mater. Sci. Eng. C 2019, 102, 589–605. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Chávez, J.J.; López-Cervantes, M.; Naïk, A.; Kalia, Y.N.; Quintanar-Guerrero, D.; Ganem-Quintanar, A. Applications of thermo-reversible pluronic F-127 gels in pharmaceutical formulations. J. Pharm. Pharm. Sci. 2006, 9, 339–358. [Google Scholar]
- Qiu, Y.; Hamilton, S.K.; Temenoff, J. Improving mechanical properties of injectable polymers and composites. In Injectable Biomaterials Science and Applications. Woodhead Publishing Series in Biomaterials; Elsevier: Amsdterdam, The Netherlands, 2011; pp. 61–91. [Google Scholar] [CrossRef]
- Wang, W.; Wat, E.; Hui, P.C.L.; Chan, B.; Ng, F.S.F.; Kan, C.; Wang, X.; Hu, H.; Wong, E.C.W.; Lau, C.B.S.; et al. Dual-functional transdermal drug delivery system with controllable drug loading based on thermosensitive poloxamer hydrogel for atopic dermatitis treatment. Sci. Rep. 2016, 6, 24112. [Google Scholar] [CrossRef] [Green Version]
- Derakhshandeh, K.; Fashi, M.; Seifoleslami, S. Thermosensitive pluronic® hydrogel: Prolonged injectable formulation for drug abuse. Drug Des. Devel. Ther. 2010, 4, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.K.; Won, Y.W.; Lim, K.S.; Kim, Y.H. Low-molecular-weight methylcellulose-based thermo-reversible gel/pluronic micelle combination system for local and sustained docetaxel delivery. Pharm. Res. 2012, 29, 525–534. [Google Scholar] [CrossRef]
- Chen, X.; Song, Z.; Li, S.; Tat Thang, N.; Gao, X.; Gong, X.; Guo, M. Facile one-pot synthesis of self-assembled nitrogen-doped carbon dots/cellulose nanofibril hydrogel with enhanced fluorescence and mechanical properties. Green Chem. 2020, 22, 3296–3308. [Google Scholar] [CrossRef]
- Alammar, A.; Park, S.H.; Ibrahim, I.; Deepak, A.; Holtzl, T.; Dumée, L.F.; Lim, H.N.; Szekely, G. Architecting neonicotinoid-scavenging nanocomposite hydrogels for environmental remediation. Appl. Mater. Today 2020, 21, 100878. [Google Scholar] [CrossRef]
- Yuan, J.; Yi, C.; Jiang, H.; Liu, F.; Cheng, G.J. Direct ink writing of hierarchically porous cellulose/alginate monolithic hydrogel as a highly effective adsorbent for environmental applications. ACS Appl. Polym. Mater. 2021, 3, 699–709. [Google Scholar] [CrossRef]
- Haan, T.Y.; Mohd Syahmi Hafizi Ghani, M.A.W. Physical and Chemical Cleaning for Nanofiltration/Reverse Osmosis (NF/RO) Membranes in Treatment of Tertiary Palm Oil Mill Effluent (POME) for Water Reclamation. J. Kejuruter. 2018, 1, 51–58. [Google Scholar] [CrossRef]
- Norwana, A.A.B.D.; Kunjappan, R.; Chin, M.; Schoneveld, G.; Potter, L.; Andriani, R. Center for International Forestry Research, Indonesia. The local impacts of oil palm expansion in Malaysia An assessment based on a case study in Sabah State. CIFOR Working Paper 2011, 78, 1–17. [Google Scholar]
- Haan, T.Y.; Takriff, M.S. Zero waste technologies for sustainable development in palm oil mills. J. Oil Palm. Environ. Health 2021, 12, 55–68. [Google Scholar] [CrossRef]
- Hamzah, N.; Tokimatsu, K.; Yoshikawa, K. Solid fuel from oil palm biomass residues and municipal solid waste by hydrothermal treatment for electrical power generation in Malaysia: A review. Sustainability 2019, 11, 1060. [Google Scholar] [CrossRef] [Green Version]
- Haan, Y.; Norashiqin, S.; Chun, K. Sustainable approach to the synthesis of cellulose membrane from oil palm empty fruit bunch for dye wastewater treatment. J. Water Process Eng. 2020, 34, 1–9. [Google Scholar] [CrossRef]
- Sudiyani, Y.; Styarini, D.; Triwahyuni, E. Utilization of biomass waste empty fruit bunch fiber of palm oil for bioethanol production using pilot–scale unit. Energy Procedia 2013, 32, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Nazir, M.S.; Wahjoedi, B.A.; Yussof, A.W.; Abdullah, M.A. Eco-friendly extraction and characterization of cellulose from oil palm empty fruit bunches. BioResources 2013, 8, 2161–2172. [Google Scholar] [CrossRef] [Green Version]
- Schmolka, I.R. Artificial Skin, I. Preparation and Properties of Pluronic F-127 Gels for Treatment of Burns. J. Biomed. Mater. Res. 1972, 6, 571–582. [Google Scholar] [CrossRef]
- Nie, S.; Hsiao, W.W.; Pan, W.; Yang, Z. Thermoreversible pluronic® f127-based hydrogel containing liposomes for the controlled delivery of paclitaxel: In vitro drug release, cell cytotoxicity, and uptake studies. Int. J. Nanomedicine 2011, 6, 151–166. [Google Scholar] [CrossRef] [Green Version]
- Dewan, M.; Sarkar, G.; Bhowmik, M.; Das, B.; Chattoapadhyay, A.K.; Rana, D.; Chattopadhyay, D. Effect of gellan gum on the thermogelation property and drug release profile of poloxamer 407 based ophthalmic formulation. Int. J. Biol. Macromol. 2017, 102, 258–265. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, F.C.; Calixto, G.; Hatakeyama, I.N.; Luz, G.M.; Gremião, M.P.D.; Chorilli, M. Rheological, mechanical, and bioadhesive behavior of hydrogels to optimize skin delivery systems. Drug Dev. Ind. Pharm. 2013, 39, 1750–1757. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Sun, H.; Wang, Y.; Sánchez-Soto, M.; Schiraldi, D. The relation between the rheological properties of gels and the mechanical properties of their corresponding aerogels. Gels 2018, 4, 33. [Google Scholar] [CrossRef] [Green Version]
- Sudipta, C.; Hui, P.C.; Kan, C.; Wan, W. Dual-responsive (pH/temperature) pluronic f-127 hydrogel drug delivery system for textile-based transdermal therapy. Sci. Rep. 2019, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Stojkovska, J.; Djurdjevic, Z.; Jancic, I.; Bufan, B.; Milenkovic, M.; Jankovic, R.; Miskovic-Stankovic, V.; Obradovic, B. Comparative in vivo evaluation of novel formulations based on alginate and silver nanoparticles for wound treatments. J. Biomater. Appl. 2018, 32, 1197–1211. [Google Scholar] [CrossRef] [PubMed]
- Morsi, N.M.; Abdelbary, G.A.; Ahmed, M.A. Silver sulfadiazine based cubosome hydrogels for topical treatment of burns: Development and in vitro/in vivo characterization. Eur. J. Pharm. Biopharm. 2014, 86, 178–189. [Google Scholar] [CrossRef] [PubMed]
- Al-Rajabi, M.M.; Haan, T.Y. Influence of vertical diffusion cell set-up on in vitro silver sulfadiazine drug release from thermo-responsive cellulose hydrogel. Mater. Sci. Forum 2021, 1030, 19–26. [Google Scholar] [CrossRef]
- Marques, M.; Ueda, C.T.; Shah, V.P.; Derdzinski, K.; Ewing, G.; Flynn, G.; Maibach, H.; Rytting, H.; Shaw, S.; Thakker, K.; et al. Topical and transdermal drug products. Pharmacopeial Forum 2009, 35, 750–764. [Google Scholar]
- Deo, S.S.; Inam, F.; Karmarkar, N.P. Analytical method development for determination of performance of adapalene in adapalene 0.1% gel formulation using manual diffusion cell. Chem. Sci. Trans. 2012, 2, 251–257. [Google Scholar] [CrossRef] [Green Version]
- Mustafa, F.M.; Hodali, H.A. Use of mesoporous silicate nanoparticles as drug carrier for mefenamic acid. IOP Conf. Ser. Mater. Sci. Eng. 2015, 92. [Google Scholar] [CrossRef]
- Varelas, C.G.; Dixon, D.G.; Steiner, C.A. Zero-order release from biphasic polymer hydrogels. J. Control. Release 1995, 34, 185–192. [Google Scholar] [CrossRef]
- Bruschi, M.L. Mathematical models of drug release. In Strategies to Modify the Drug Release from Pharmaceutical Systems; Elsevier: Amsterdam, The Netherlands, 2015; pp. 63–86. [Google Scholar] [CrossRef]
- Siepmann, J.; Peppas, N.A. Higuchi equation: Derivation, applications, use and misuse. Int. J. Pharm. 2011, 418, 6–12. [Google Scholar] [CrossRef]
- Korsmeyer, R.W.; Gurny, R.; Doelker, E.; Buri, P.; Peppas, N.A. Mechanisms of solute release from porous hydrophilic polymers. Int. J. Pharm. 1983, 15, 25–35. [Google Scholar] [CrossRef]
- Gioffredi, E.; Boffito, M.; Calzone, S.; Giannitelli, S.M.; Rainer, A.; Trombetta, M.; Mozetic, P.; Chiono, V. Pluronic F127 hydrogel characterization and biofabrication in cellularized constructs for tissue engineering applications. Procedia CIRP 2016, 49, 125–132. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.; Seok, P.W.; Park, H.; Lee, D.K.; Na, K. Thermo-sensitive injectable hydrogel based on the physical mixing of hyaluronic acid and pluronic f-127 for sustained NSAID delivery. Carbohydr. Polym. 2017, 156, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Pereira, G.G.; Dimer, F.A.; Guterres, S.S.; Kechinski, C.P.; Granada, J.E.; Cardozo, N.S.M. Formulation and characterization of poloxamer 407®: Thermoreversible gel containing polymeric microparticles and hyaluronic acid. Quim. Nov. 2013, 36, 1121–1125. [Google Scholar] [CrossRef] [Green Version]
- Dragicevic, N.; Maibach, H.I. Polymeric micelles in dermal and transdermal delivery. In Percutaneous Penetration Enhancers Chemical Methods in Penetration Enhancement: Nanocarriers; Springer: Berlin, Germany, 2016; pp. 1–384. [Google Scholar] [CrossRef]
- Katas, H.; Thian Sian, T.; Abdul Ghaf, M. Topical temperature-sensitive gel containing DsiRNA-chitosan nanoparticles for potential treatment of skin cancer. Trends Med. Res. 2017, 12, 1–13. [Google Scholar] [CrossRef]
- Hopkins, C.C.; de Bruyn, J.R. Gelation and long-time relaxation of aqueous solutions of pluronic f127. J. Rheol. 2019, 63, 191–201. [Google Scholar] [CrossRef]
- Tae, G.; Won, D. Composition for Forming Pluronic-Based Hydrogel With Improved Stability. US Patent 2015/0231246 A1, 19 February 2015. [Google Scholar]
- Garala, K.; Joshi, P.; Patel, J.; Ramkishan, A.; Shah, M. Formulation and evaluation of periodontal in situ gel. Int. J. Pharm. Investig. 2013, 3, 29. [Google Scholar] [CrossRef] [Green Version]
- Orasugh, J.T.; Sarkar, G.; Saha, N.R.; Das, B.; Bhattacharyya, A.; Das, S.; Mishra, R.; Roy, I.; Chattoapadhyay, A.; Ghosh, S.K.; et al. Effect of cellulose nanocrystals on the performance of drug loaded in situ gelling thermo-responsive ophthalmic formulations. Int. J. Biol. Macromol. 2019, 124, 235–245. [Google Scholar] [CrossRef]
- Gong, C.Y.; Shi, S.; Dong, P.W.; Zheng, X.L.; Fu, S.Z.; Guo, G.; Yang, J.L.; Wei, Y.Q.; Qian, Z.Y. In vitro drug release behavior from a novel thermosensitive composite hydrogel based on pluronic f127 and poly(ethylene glycol)-poly(ε-caprolactone)-poly(ethylene glycol) copolymer. BMC Biotechnol. 2009, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Omri, M.A.; Triki, A.; Guicha, M.; Hassen, M.; Ben, A.M.; Ahmed El Hamzaoui, H.; Bulou, A. Effect of wool and thermo-binder fibers on adhesion of alfa fibers in polyester composite. J. Appl. Phys. 2013, 114. [Google Scholar] [CrossRef]
- Teow, Y.; Ming, K.; Mohammad, A. Synthesis of cellulose hydrogel for copper (II) ions adsorption. J. Environ. Chem. Eng. 2018, 6, 4588–4597. [Google Scholar] [CrossRef]
- Prasad, S.G.; De, A.; De, U. Structural and optical investigations of radiation damage in transparent PET polymer films. Int. J. Spectrosc. 2011, 2011. [Google Scholar] [CrossRef] [Green Version]
- Liu, T.; Jiao, C.; Peng, X.; Chen, Y.-N.; Chen, Y.; He, C.; Liu, R.; Wang, H. Super-strong and tough poly(vinyl alcohol)/poly(acrylic acid) hydrogels reinforced by hydrogen bonding. J. Mater. Chem. B 2018, 8105–8114. [Google Scholar] [CrossRef]
- Rasoulzadeh, M.; Namazi, H. Carboxymethyl cellulose/graphene oxide bio-nanocomposite hydrogel beads as anticancer drug carrier agent. Carbohydr. Polym. 2017, 168, 320–326. [Google Scholar] [CrossRef]
- Mastropietro, D.J.; Nimroozi, R.; Omidian, H. Rheology in pharmaceutical formulations-A perspective. J. Dev. Drugs 2013, 2, 2–7. [Google Scholar] [CrossRef] [Green Version]
- Grassi, G.; Crevatin, A.; Farra, R.; Guarnieri, G.; Pascotto, A.; Rehimers, B.; Lapasin, R.; Grassi, M. Rheological properties of aqueous pluronic-alginate systems containing liposomes. J. Colloid Interface Sci. 2006, 301, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Owczarz, P.; Zi, P.; Modrzejewska, Z.; Kuberski, S.; Dziubinski, M. Rheo-kinetic study of sol-gel phase transition of chitosan colloidal systems. Polymers 2018, 10, 47. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fakhari, A.; Corcoran, M.; Schwarz, A. Thermogelling properties of purified poloxamer 407. Heliyon 2017, 3, e00390. [Google Scholar] [CrossRef]
- Bobbala, S.; Tamboli, V.; McDowell, A.; Mitra, A.K.; Hook, S. Novel injectable pentablock copolymer based thermoresponsive hydrogels for sustained release vaccines. AAPS J. 2016, 18, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Hsu, S.H.; Leu, Y.L.; Hu, J.W.; Fang, J.Y. Physicochemical characterization and drug release of thermosensitive hydrogels composed of a hyaluronic acid/pluronic f127 graft. Chem. Pharm. Bull. 2009, 57, 453–458. [Google Scholar] [CrossRef] [Green Version]
- Wani, T.U.; Rashid, M.; Kumar, M.; Chaudhary, S.; Kumar, P.; Mishra, N. Targeting aspects of nanogels: An overview. Int. J. Pharm. Sci. Nanotech. 2014, 7, 2612–2630. [Google Scholar]
- Gupta, A.; Kowalczuk, M.; Heaselgrave, W.; Britland, S.T.; Martin, C.; Radecka, I. The production and application of hydrogels for wound management: A review. Eur. Polym. J. 2018, 111, 134–151. [Google Scholar] [CrossRef]
- Flory, P.J.; Rehner, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 1943, 11, 521–526. [Google Scholar] [CrossRef]
- Vervoort, S. Behaviour of hydrogels swollen in polymer solutions under mechanical action. Ph.D. Thesis, École Nationale Supérieure des Mines de Paris, Paris, France, 2006. [Google Scholar]
- Sakai, T. Swelling and deswelling. In Physics of Polymer Gels; Wiley-VCHVerlag GmbH& Co. KGaA.: Weinheim, Germany, 2020; pp. 77–107. [Google Scholar]
- Hillery, A.M.; Park, K. Drug Delivery: Fundamentals and Applications, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar] [CrossRef]
- Siqueira, N.M.; Cirne, M.F.R.; Immich, M.F.; Poletto, F. Stimuli-responsive polymeric hydrogels and nanogels for drug delivery applications. In Stimuli Responsive Polymeric Nanocarriers for Drug Delivery Applications; Elsevier Ltd.: Amsdterdam, The Netherlands, 2018; Volume 1, pp. 343–374. [Google Scholar] [CrossRef]
- Jodar, K.S.P.; Balcão, V.M.; Chaud, M.V.; Tubino, M.; Yoshida, V.M.H.; Oliveira, J.M.; Vila, M.M.D.C. Development and characterization of a hydrogel containing silver sulfadiazine for antimicrobial topical applications. J. Pharm. Sci. 2015, 104, 2241–2254. [Google Scholar] [CrossRef] [Green Version]
- Huang, S.; Fu, X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J. Control. Release 2010, 142, 149–159. [Google Scholar] [CrossRef]
- Thakkar, V.; Korat, V.; Baldaniya, L.; Gohel, M.; Gandhi, T.; Patel, N. Development and characterization of novel hydrogel containing antimicrobial drug for treatment of burns. Int. J. Pharm. Investig. 2016, 6, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalaydina, R.V.; Bajwa, K.; Qorri, B.; Decarlo, A.; Szewczuk, M.R. Recent advances in “smart” delivery systems for extended drug release in cancer therapy. Int. J. Nanomedicine 2018, 13, 4727–4745. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, P.K. Principles and basic concepts of toxicokinetics. In Fundamentals of Toxicology; Academic Press: Waltham, MA, USA, 2016; pp. 87–107. [Google Scholar] [CrossRef]
- Nascimento, E.G.D.; Sampaio, T.B.M.; Medeiros, A.C.; Azevedo, E.P.D. Evaluation of chitosan gel with 1% silver sulfadiazine as an alternative for burn wound treatment in rats. Acta Cirúrgica Bras. 2009, 24, 2009–2460. [Google Scholar]
- Church, D.; Elsayed, S.; Reid, O.; Winston, B.; Lindsay, R. Burn wound infections. Clin. Microbiol. Rev. 2006, 19, 403–434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dorota, W.; Krzak, J.; Macikowski, B.; Berkowski, R.; Osinski, B.; Musiał, W. Evaluation of the release kinetics of a pharmacologically active substance from model intra-articular implants replacing the cruciate ligaments of the knee. Materials (Basel) 2019, 12, 1202. [Google Scholar] [CrossRef] [Green Version]
- Wong, R.S.H.; Dodou, K. Effect of drug loading method and drug physicochemical properties on the material and drug release properties of poly (ethylene oxide) hydrogels for transdermal delivery. Polymers 2017, 9, 286. [Google Scholar] [CrossRef] [PubMed]
- Olejnik, A.; Glowka, A.; Nowak, I. Release studies of undecylenoyl phenylalanine from topical formulations. Saudi Pharm. J. 2018, 26, 709–718. [Google Scholar] [CrossRef]
- Rabin, C.R.; Siegel, S.J. Delivery systems and dosing for antipsychotics. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2012; Volume 212, pp. 267–291. [Google Scholar]


| Sample | PF127 Polymer (w/v%) | Cellulose Fibers (w/v%) | DI Water (w/v%) |
|---|---|---|---|
| H1 | 15 | 0.0 | 85 |
| H2 | 1.0 | 84 | |
| H3 | 2.0 | 83 | |
| H4 | 3.0 | 82 | |
| H5 | 20 | 0.0 | 80 |
| H6 | 1.0 | 79 | |
| H7 | 2.0 | 78 | |
| H8 | 3.0 | 77 | |
| H9 | 25 | 0.0 | 75 |
| H10 | 1.0 | 74 | |
| H11 | 2.0 | 73 | |
| H12 | 3.0 | 72 | |
| H13 | 30 | 0.0 | 70 |
| H14 | 1.0 | 69 | |
| H15 | 2.0 | 68 | |
| H16 | 3.0 | 67 | |
| H17 | 35 | 0.0 | 65 |
| H18 | 1.0 | 64 | |
| H19 | 2.0 | 63 | |
| H20 | 3.0 | 62 |
| Sample | LCST (°C) | UCST (°C) | Status at (4 °C) | Status at (20 °C) | Status at (37 °C) |
|---|---|---|---|---|---|
| H1 | N/D | N/D | - | - | - |
| H2 | N/D | N/D | - | + | + |
| H3 | N/D | N/D | + | + | ++ |
| H4 | N/D | N/D | + | ++ | ++ |
| H5 | 24.0 ± 1.0 | 58.3 ± 2.9 | - | + | +++ |
| H6 | 23.7 ± 0.6 | 61.7 ± 2.9 | + | + | +++ |
| H7 | 22.3 ± 1.2 | 68.3 ± 2.9 | + | ++ | +++ |
| H8 | 21.0 ± 1.0 | 78.3 ± 2.9 | + | ++ | +++ |
| H9 | 20.0 ± 1.0 | 73.3 ± 2.9 | - | +++ | +++ |
| H10 | 17.0 ± 1.0 | 78.3 ± 2.9 | + | +++ | +++ |
| H11 | 15 ± 0.0 | 86.7 ± 2.9 | + | +++ | +++ |
| H12 | <15 | N/D | ++ | +++ | +++ |
| H13 | 17.7 ± 0.6 | N/D | - | +++ | +++ |
| H14 | 15 ± 0.0 | N/D | + | +++ | +++ |
| H15 | <15 | N/D | ++ | +++ | +++ |
| H16 | <15 | N/D | ++ | +++ | +++ |
| H17 | 16.7 ± 0.6 | N/D | - | +++ | +++ |
| H18 | <15 | N/D | + | +++ | +++ |
| H19 | <15 | N/D | ++ | +++ | +++ |
| H20 | <15 | N/D | ++ | +++ | +++ |
| Hydrogel | Zero-Order | First-Order | Higuchi | Korsmeyer-Peppas | |||||
|---|---|---|---|---|---|---|---|---|---|
| k0 | R2 | k1 | R2 | kH | R2 | kr | n | R2 | |
| H5 | 5.583 | 0.980 | 0.140 | 0.954 | 26.662 | 0.968 | 10.839 | 0.727 | 0.994 |
| H6 | 2.708 | 0.986 | 0.048 | 0.954 | 15.568 | 0.955 | 7.129 | 0.671 | 0.991 |
| H7 | 2.585 | 0.985 | 0.041 | 0.946 | 14.330 | 0.923 | 5.023 | 0.710 | 0.967 |
| H8 | 1.762 | 0.987 | 0.023 | 0.969 | 10.046 | 0.940 | 9.462 | 0.746 | 0.976 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al-Rajabi, M.M.; Teow, Y.H. Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery. Polymers 2021, 13, 2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132153
Al-Rajabi MM, Teow YH. Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery. Polymers. 2021; 13(13):2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132153
Chicago/Turabian StyleAl-Rajabi, Maha Mohammad, and Yeit Haan Teow. 2021. "Green Synthesis of Thermo-Responsive Hydrogel from Oil Palm Empty Fruit Bunches Cellulose for Sustained Drug Delivery" Polymers 13, no. 13: 2153. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132153






