Antimicrobial Polymeric Composites with Embedded Nanotextured Magnesium Oxide
Abstract
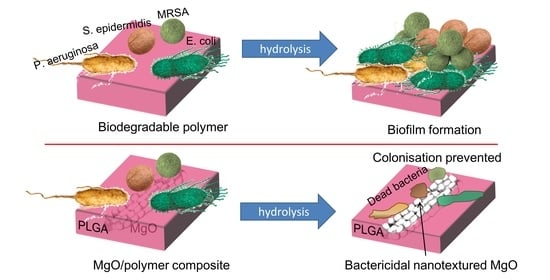
:1. Introduction
2. Materials and Methods
2.1. Synthesis and Processing
2.2. Composite Characterization
2.3. Magnesium Release from MgO/Polymer Composites
2.4. Quantification of Released Magnesium
2.5. Characterisation of Composites after Exposure to Physiological Solution
2.6. Metabolic Activity of Attached Bacteria
2.7. Viability of Attached Bacteria
2.8. Lethal Potential for Planktonic Bacteria
2.9. Haemolysis Assay
2.10. Morphology of RBCs or Bacteria on Coatings
2.11. Statistical Analysis
3. Results
3.1. Structural Properties of MgO/Polymer Composites
3.2. Release of MgO MRs from the MgO/Polymer Composites under In Vitro Physiological Conditions
3.3. Antibacterial Properties of the MgO/Polymer Composite Coatings
3.4. The Impact of the MgO/PLGA Composites on Red Blood Cells
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Castiglioni, S.; Cazzaniga, A.; Albisetti, W.; Maier, J.A.M. Magnesium and Osteoporosis: Current State of Knowledge and Future Research Directions. Nutrients 2013, 5, 3022–3033. [Google Scholar] [CrossRef] [Green Version]
- Rude, R.K.; Gruber, H.E.; Norton, H.J.; Wei, L.Y.; Frausto, A.; Kilburn, J. Reduction of dietary magnesium by only 50% in the rat disrupts bone and mineral metabolism. Osteoporos. Int. 2006, 17, 1022–1032. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Feyerabend, F.; Schilling, A.F.; Willumeit-Römer, R.; Luthringer, B.J.C. Effects of extracellular magnesium extract on the proliferation and differentiation of human osteoblasts and osteoclasts in coculture. Acta Biomater. 2015, 27, 294–304. [Google Scholar] [CrossRef] [PubMed]
- Hickey, D.J.; Ercan, B.; Sun, L.; Webster, T.J. Adding MgO nanoparticles to hydroxyapatite-PLLA nanocomposites for improved bone tissue engineering applications. Acta Biomater. 2015, 14, 175–184. [Google Scholar] [CrossRef] [PubMed]
- Zreiqat, H.; Howlett, C.R.; Zannettino, A.; Evans, P.; Schulze-Tanzil, G.; Knabe, C.; Shakibaei, M. Mechanisms of magnesium-stimulated adhesion of osteoblastic cells to commonly used orthopaedic implants. J. Biomed. Mater. Res. 2002, 62, 175–184. [Google Scholar] [CrossRef]
- Fiocco, L.; Li, S.; Stevens, M.M.; Bernardo, E.; Jones, J.R. Biocompatibility and bioactivity of porous polymer-derived Ca-Mg silicate ceramics. Acta Biomater. 2017, 50, 56–67. [Google Scholar] [CrossRef]
- Zhai, W.; Lu, H.; Wu, C.; Chen, L.; Lin, X.; Naoki, K.; Chen, G.; Chang, J. Stimulatory effects of the ionic products from Ca-Mg-Si bioceramics on both osteogenesis and angiogenesis in vitro. Acta Biomater. 2013, 9, 8004–8014. [Google Scholar] [CrossRef]
- Kum, C.H.; Cho, Y.; Seo, S.H.; Joung, Y.K.; Ahn, D.J.; Han, D.K. A poly(lactide) stereocomplex structure with modified magnesium oxide and its effects in enhancing the mechanical properties and suppressing inflammation. Small 2014, 10, 3783–3794. [Google Scholar] [CrossRef]
- Anicic, N.; Vukomanovic, M.; Suvorov, D. The Nano-Texturing of MgO Microrods for Antibacterial Applications. RSC Adv. 2016, 6, 102657–102664. [Google Scholar] [CrossRef]
- Leung, Y.H.; Ng, A.M.C.; Xu, X.; Shen, Z.; Gethings, L.A.; Wong, M.T.; Chan, C.M.N.; Guo, M.Y.; Ng, Y.H.; Djurisic, A.B.; et al. Mechanisms of Antibacterial Activity of MgO: Non-ROS Mediated Toxicity of MgO Nanoparticles Towards Escherichia Coli. Small 2014, 10, 1171–1183. [Google Scholar] [CrossRef] [PubMed]
- Sawai, J.; Yoshikawa, T. Quantitative Evaluation of Antifungal Activity of Metallic Oxide Powders (MgO, CaO and ZnO) by an Indirect Conductimetric Assay. J. Appl. Microbiol. 2004, 96, 803–809. [Google Scholar] [CrossRef]
- Aničić, N.; Vukomanović, M.; Koklič, T.; Suvorov, D. Fewer Defects in the Surface Slows the Hydrolysis Rate, Decreases the ROS Generation Potential, and Improves the Non-ROS Antimicrobial Activity of MgO. Small 2018, 14, 1800205. [Google Scholar] [CrossRef]
- Wetteland, C.L.; Nguyen, N.-Y.T.; Liu, H. Concentration-Dependent Behaviors of Bone Marrow Derived Mesenchymal Stem Cells and Infectious Bacteria Toward Magnesium Oxide Nanoparticles. Acta Biomater. 2016, 35, 341–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gristina, A.G. Biomaterial-centered infection: microbial adhesion versus tissue integration. Science 1987, 237, 1588–1595. [Google Scholar] [CrossRef]
- Song, Z.; Borgwardt, L.; Hoiby, N.; Wu, H.; Sorensen, T.S.; Borgwardt, A. Prosthesis Infections after Orthopedic Joint Replacement: The Possible Role of Bacterial Biofilms. Orthop. Rev. (Pavia) 2013, 5, 65–71. [Google Scholar] [CrossRef] [Green Version]
- Arciola, C.R.; Campoccia, D.; Speziale, P.; Montanaro, L.; Costerton, J.W. Biofilm Formation in Staphylococcus Implant Infections. A Review of Molecular Mechanisms and Implications for Biofilm-Resistant Materials. Biomaterials 2012, 33, 5967–5982. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.; Monteiro, F.J.; Ferraz, M.P. Infection of orthopedic implants with emphasis on bacterial adhesion process and techniques used in studying bacterial-material interactions. Biomatter 2012, 2, 176–194. [Google Scholar] [CrossRef] [Green Version]
- Kumari, A.; Yadav, S.K.; Yadav, S.C. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf. B Biointerfaces 2010, 75, 1–18. [Google Scholar] [CrossRef]
- Makadia, H.K.; Siegel, S.J. Poly Lactic-co-Glycolic Acid (PLGA) as Biodegradable Controlled Drug Delivery Carrier. Polymers (Basel) 2011, 3, 1377–1397. [Google Scholar] [CrossRef] [PubMed]
- Gomez, E.; Estela, J.M.; Cerda, V. Simultaneous Spectrophotometric Determination of Calcium and Magnesium in Water. Anal. Chim. Acta 1991, 249, 513–518. [Google Scholar] [CrossRef]
- Sarker, S.D.; Nahar, L.; Kumarasamy, Y. Microtitre Plate-Based Antibacterial Assay Incorporating Resazurin as an Indicator of Cell Growth and its Application in the in vitro Antibacterial Screening of Phytochemicals. Methods 2007, 42, 321–324. [Google Scholar] [CrossRef] [PubMed]
- Spence, M.T.Z.; Johnson, I.D. The Molecular Probes Handbook: A Guide to Fluorescent Probes and Labeling Technologies, 11th ed.; Life Technologies Corporation: Carlsbad, CA, USA, 2010. [Google Scholar]
- Asharani, P.V.; Sethu, S.; Vadukumpully, S.; Zhong, S.; Lim, C.T.; Hande, M.P.; Valiyaveettil, S. Investigations on the Structural Damage in Human Erythrocytes Exposed to Silver, Gold and Platinum Nanoparticles. Adv. Funct. Mater. 2010, 20, 1233–1242. [Google Scholar] [CrossRef]
- Hanna, R. Infrared Properties of Magnesium Oxide. J. Am. Ceram. Soc. 1965, 48, 376–380. [Google Scholar] [CrossRef]
- Thoms, H.; Epple, M.; Reller, A. The thermal decomposition of magnesium alcoholates to magnesia (MgO): Studies by IR and thermal analysis. Solid State Ionics 1997, 101–103, 79–84. [Google Scholar] [CrossRef]
- Jevtic, M.; Radulovic, A.; Ignjatovic, N.; Mitric, M.; Uskokovic, D. Controlled Assembly of Poly(d,l-Lactide-co-Glycolide)/Hydroxyapatite Core-Shell Nanospheres under Ultrasonic Irradiation. Acta Biomater. 2009, 5, 208–218. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Webster, F.X.; Kiemle, D. Spectrometric Identification of Organic Compounds, 7th ed.; Wiley: Hoboken, NJ, USA, 2005; ISBN 1118311655. [Google Scholar]
- Elzein, T.; Nasser-Eddine, M.; Delaite, C.; Bistac, S.; Dumas, P. FTIR Study of Polycaprolactone Chain Organization at Interfaces. J. Colloid Interface Sci. 2004, 273, 381–387. [Google Scholar] [CrossRef] [PubMed]
- Marczenko, Z.; Balcerzak, M. Magnesium. In Separation, Preconcentration and Spectrophotometry in Inorganic Analysis; Elsevier: Amsterdam, The Netherlands, 2000; Volume 10, pp. 247–252. [Google Scholar]
- Scott, G. Degradable Polymers: Principles and Applications, 2nd ed.; Springer Science & Business Media: Berlin, Germany, 2002; ISBN 978-90-481-6091-4. [Google Scholar]
- Sawai, J.; Kojima, H.; Igarashi, H.; Hashimoto, A.; Shoji, S.; Sawaki, T.; Hakoda, A.; Kawada, E.; Kokugan, T.; Shimizu, M. Antibacterial Characteristics of Magnesium Oxide Powder. World J. Microbiol. Biotechnol. 2000, 16, 187–194. [Google Scholar] [CrossRef]
- Crouzet, M.; Le Senechal, C.; Brözel, V.S.; Costaglioli, P.; Barthe, C.; Bonneu, M.; Garbay, B.; Vilain, S. Exploring early steps in biofilm formation: Set-up of an experimental system for molecular studies. BMC Microbiol. 2014, 14, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiptipakorn, S.; Keungputpong, N.; Phothiphiphit, S.; Rimdusit, S. Effects of polycaprolactone molecular weights on thermal and mechanical properties of polybenzoxazine. J. Appl. Polym. Sci. 2015, 132, 1–11. [Google Scholar] [CrossRef]
- Campoccia, D.; Montanaro, L.; Arciola, C.R. The significance of infection related to orthopedic devices and issues of antibiotic resistance. Biomaterials 2006, 27, 2331–2339. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Webster, T.J. Bacteria antibiotic resistance: New challenges and opportunities for implant-associated orthopedic infections. J. Orthop. Res. 2018, 36, 22–32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Londono, R.; Badylak, S.F. Factors Which Affect the Host Response to Biomaterials. In Host Response to Biomaterials: The Impact of Host Response on Biomaterial Selection; Badylak, S.F., Ed.; Elsevier Inc.: San Diego, CA, USA, 2015; pp. 1–12. ISBN 9780128005002. [Google Scholar]
- Li, X.; Chu, C.; Wei, Y.; Qi, C.; Bai, J.; Guo, C.; Xue, F.; Lin, P.; Chu, P.K. In vitro degradation kinetics of pure PLA and Mg/PLA composite: Effects of immersion temperature and compression stress. Acta Biomater. 2017, 48, 468–478. [Google Scholar] [CrossRef]
- Lim, G.H.W.; Wortis, M.; Mukhopadhyay, R. Stomatocyte-Discocyte-Echinocyte Sequence of the Human Red Blood Cell: Evidence for the Bilayer- Couple Hypothesis from Membrane Mechanics. Proc. Natl. Acad. Sci. USA 2002, 99, 16766–16769. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Liu, B.; You, C.; Chen, M. Effects of MgO whiskers on mechanical properties and crystallization behavior of PLLA/MgO composites. Mater. Des. 2016, 89, 573–581. [Google Scholar] [CrossRef]
- Wen, W.; Zou, Z.; Luo, B.; Zhou, C. In vitro degradation and cytocompatibility of g-MgO whiskers/PLLA composites. J. Mater. Sci. 2017, 52, 2329–2344. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, B.; Bi, H.; Yang, J.; Li, W.; Liang, H.; Liang, Y.; Jia, Z.; Shi, S.; Chen, M. The degradation properties of MgO whiskers/PLLA composite in vitro. Int. J. Mol. Sci. 2018, 19, 2740. [Google Scholar] [CrossRef] [PubMed] [Green Version]









Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aničić, N.; Kurtjak, M.; Jeverica, S.; Suvorov, D.; Vukomanović, M. Antimicrobial Polymeric Composites with Embedded Nanotextured Magnesium Oxide. Polymers 2021, 13, 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132183
Aničić N, Kurtjak M, Jeverica S, Suvorov D, Vukomanović M. Antimicrobial Polymeric Composites with Embedded Nanotextured Magnesium Oxide. Polymers. 2021; 13(13):2183. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132183
Chicago/Turabian StyleAničić, Nemanja, Mario Kurtjak, Samo Jeverica, Danilo Suvorov, and Marija Vukomanović. 2021. "Antimicrobial Polymeric Composites with Embedded Nanotextured Magnesium Oxide" Polymers 13, no. 13: 2183. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13132183