Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices—A Systematic Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Polymer Films via Spray Coating
2.3. Raman Microscopy Imaging
2.4. FTIR Measurements
2.5. SEM Imaging
2.6. Contact Angle Measurements
2.7. Thermal Analysis
2.8. Mechanical Testsing
2.9. Statistical Analysis
3. Results
3.1. Spectroscopical Analysis of Drug Loaded PLLA Films
3.2. Influence of Drug Incorporation on the Surface Morphology and Drug Distribution
3.3. Influence of Drug Incorporation on Surface Hydrophilicity
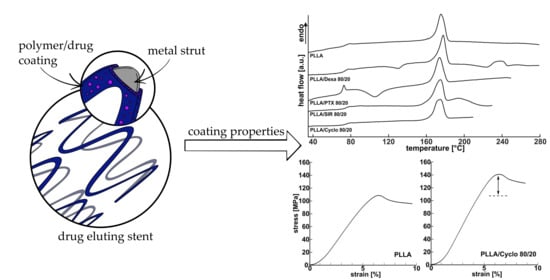
3.4. Influence of Drug Incorporation on the Thermal Properties—DSC
3.5. Coating Thickness Determination
3.6. Influence of Drug Incorporation on the Mechanical Properties
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Seidlitz, A. Drug-Eluting Stents. In In vitro Drug Release Testing of Special Dosage Forms, 1st ed.; Fotaki, N., Klein, S., Eds.; Wiley: Hoboken, NJ, USA, 2020; pp. 87–117. ISBN 9781118341476. [Google Scholar]
- Torii, S.; Jinnouchi, H.; Sakamoto, A.; Kutyna, M.; Cornelissen, A.; Kuntz, S.; Guo, L.; Mori, H.; Harari, E.; Paek, K.H.; et al. Drug-eluting coronary stents: Insights from preclinical and pathology studies. Nat. Rev. Cardiol. 2020, 17, 37–51. [Google Scholar] [CrossRef] [PubMed]
- Ali, R.M.; Abdul Kader, M.; Wan Ahmad, W.A.; Ong, T.K.; Liew, H.B.; Omar, A.-F.; Mahmood Zuhdi, A.S.; Nuruddin, A.A.; Schnorr, B.; Scheller, B. Treatment of Coronary Drug-Eluting Stent Restenosis by a Sirolimus- or Paclitaxel-Coated Balloon. JACC Cardiovasc. Interv. 2019, 12, 558–566. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, W.; Lanzer, P.; Behrens, P.; Brandt-Wunderlich, C.; Öner, A.; Ince, H.; Schmitz, K.-P.; Grabow, N. Direct comparison of coronary bare metal vs. drug-eluting stents: Same platform, different mechanics? Eur. J. Med. Res. 2018, 23, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.J.; Way, J.A.H.; Kritharides, L.; Brieger, D. Polymer-free versus durable polymer drug-eluting stents in patients with coronary artery disease: A meta-analysis. Ann. Med. Surg. (Lond.) 2019, 38, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Von Birgelen, C.; Buiten, R.A. Superiority of biodegradable polymer sirolimus-eluting stents in STEMI. Lancet 2019, 394, 1208–1210. [Google Scholar] [CrossRef]
- Grubman, D.; Saito, Y.; Lansky, A. The Firehawk stent: A review of a novel abluminal groove-filled biodegradable polymer sirolimus-eluting stent. Argentina de Cardioangiología Intervencionista 2019, 10, 73–77. [Google Scholar] [CrossRef]
- Yoshioka, G.; Nishihira, K.; Asada, Y.; Node, K. In-stent restenosis following third-generation sirolimus-eluting stent implantation: First report analysed from imaging modalities and histopathological findings. Eur. Heart J. 2020, 17, 1707. [Google Scholar] [CrossRef]
- Bünger, C.M.; Grabow, N.; Sternberg, K.; Kröger, C.; Ketner, L.; Schmitz, K.-P.; Kreutzer, H.J.; Ince, H.; Nienaber, C.A.; Klar, E.; et al. Sirolimus-eluting biodegradable poly-L-lactide stent for peripheral vascular application: A preliminary study in porcine carotid arteries. J. Surg. Res. 2007, 139, 77–82. [Google Scholar] [CrossRef]
- Stone, G.W.; Ellis, S.G.; Cox, D.A.; Hermiller, J.; O’Shaughnessy, C.; Mann, J.T.; Turco, M.; Caputo, R.; Bergin, P.; Greenberg, J.; et al. A polymer-based, paclitaxel-eluting stent in patients with coronary artery disease. N. Engl. J. Med. 2004, 350, 221–231. [Google Scholar] [CrossRef]
- Razavi, M.K.; Donohoe, D.; D’Agostino, R.B.; Jaff, M.R.; Adams, G. Adventitial Drug Delivery of Dexamethasone to Improve Primary Patency in the Treatment of Superficial Femoral and Popliteal Artery Disease: 12-Month Results from the DANCE Clinical Trial. JACC Cardiovasc. Interv. 2018, 11, 921–931. [Google Scholar] [CrossRef]
- König, A.; Leibig, M.; Rieber, J.; Schiele, T.M.; Theisen, K.; Siebert, U.; Gothe, R.M.; Klauss, V. Randomized comparison of dexamethasone-eluting stents with bare metal stent implantation in patients with acute coronary syndrome: Serial angiographic and sonographic analysis. Am. Heart J. 2007, 153, 979.e1–979.e8. [Google Scholar] [CrossRef] [PubMed]
- Sternberg, K.; Kramer, S.; Nischan, C.; Grabow, N.; Langer, T.; Hennighausen, G.; Schmitz, K.-P. In vitro study of drug-eluting stent coatings based on poly(L-lactide) incorporating cyclosporine A—drug release, polymer degradation and mechanical integrity. J. Mater. Sci. Mater. Med. 2007, 18, 1423–1432. [Google Scholar] [CrossRef] [PubMed]
- Majewska, P.; Oledzka, E.; Sobczak, M. Overview of the latest developments in the field of drug-eluting stent technology. Biomater. Sci. 2020, 8, 544–551. [Google Scholar] [CrossRef]
- Wessely, R. New drug-eluting stent concepts. Nat. Rev. Cardiol. 2010, 7, 194–203. [Google Scholar] [CrossRef] [PubMed]
- Wessely, R.; Schömig, A.; Kastrati, A. Sirolimus and Paclitaxel on polymer-based drug-eluting stents: Similar but different. J. Am. Coll. Cardiol. 2006, 47, 708–714. [Google Scholar] [CrossRef] [Green Version]
- Vos, N.S.; Fagel, N.D.; Amoroso, G.; Herrman, J.-P.R.; Patterson, M.S.; Piers, L.H.; van der Schaaf, R.J.; Slagboom, T.; Vink, M.A. Paclitaxel-Coated Balloon Angioplasty versus Drug-Eluting Stent in Acute Myocardial Infarction: The Revelation Randomized Trial. JACC Cardiovasc. Interv. 2019, 12, 1691–1699. [Google Scholar] [CrossRef]
- Dash, A.K. The dark side of paclitaxel. Oncol. Rev. 2010, 4, 71–72. [Google Scholar] [CrossRef] [Green Version]
- Strohbach, A.; Busch, R. Polymers for Cardiovascular Stent Coatings. Int. J. Polym. Sci. 2015, 2015, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; de Scheerder, I.; Desmet, W. Dexamethasone-eluting stent: An anti-inflammatory approach to inhibit coronary restenosis. Expert Rev. Cardiovasc. Ther. 2004, 2, 653–660. [Google Scholar] [CrossRef]
- Prunotto, M.; Bacchetta, M.; Jayaraman, S.; Galloni, M.; van Eys, G.; Gabbiani, G.; Bochaton-Piallat, M.-L. Cytostatic drugs differentially affect phenotypic features of porcine coronary artery smooth muscle cell populations. FEBS Lett. 2007, 581, 5847–5851. [Google Scholar] [CrossRef]
- Jonasson, L.; Holm, J.; Hansson, G.K. Cyclosporin A inhibits smooth muscle proliferation in the vascular response to injury. Proc. Natl. Acad. Sci. USA 1988, 85, 2303–2306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Verdoia, M.; Kedhi, E.; Suryapranata, H.; Galasso, G.; Dudek, D.; de Luca, G. Polymer-Free vs. Polymer-Coated Drug-Eluting Stents for the Treatment of Coronary Artery Disease: A Meta-Analysis of 16 Randomized Trials. Cardiovasc. Revasc. Med. 2020, 21, 745–753. [Google Scholar] [CrossRef] [PubMed]
- Parker, T.; Davé, V.; Falotico, R. Polymers for drug eluting stents. Curr. Pharm. Des. 2010, 16, 3978–3988. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.-H.; La Torre Hernandez, J.M.D. The newest generation of drug-eluting stents and beyond. Eur. Cardiol. Rev. 2018, 13, 54. [Google Scholar] [CrossRef]
- Mokhena, T.C.; Mochane, M.J.; Sadiku, E.R.; Agboola, O.; John, M.J. Opportunities for PLA and Its Blends in Various Applications. In Green Biopolymers and Their Nanocomposites, 1st ed.; Gnanasekaran, D., Ed.; Springer: Singapore, 2019; pp. 55–81. ISBN 978-981-13-8063-1. [Google Scholar]
- Hu, T.; Yang, J.; Cui, K.; Rao, Q.; Yin, T.; Tan, L.; Zhang, Y.; Li, Z.; Wang, G. Controlled Slow-Release Drug-Eluting Stents for the Prevention of Coronary Restenosis: Recent Progress and Future Prospects. ACS Appl. Mater. Interfaces 2015, 11695–11712. [Google Scholar] [CrossRef]
- Nguyen, K.T.; Shaikh, N.; Wawro, D.; Zhang, S.; Schwade, N.D.; Eberhart, R.C.; Tang, L. Molecular responses of vascular smooth muscle cells to paclitaxel-eluting bioresorbable stent materials. J. Biomed. Mater. Res. A 2004, 69, 513–524. [Google Scholar] [CrossRef]
- Can, E.; Udenir, G.; Kanneci, A.I.; Kose, G.; Bucak, S. Investigation of PLLA/PCL blends and paclitaxel release profiles. AAPS PharmSciTech 2011, 12, 1442–1453. [Google Scholar] [CrossRef]
- Steele, T.W.J.; Huang, C.L.; Widjaja, E.; Boey, F.Y.C.; Loo, J.S.C.; Venkatraman, S.S. The effect of polyethylene glycol structure on paclitaxel drug release and mechanical properties of PLGA thin films. Acta Biomater. 2011, 7, 1973–1983. [Google Scholar] [CrossRef]
- Hobzova, R.; Hampejsova, Z.; Cerna, T.; Hrabeta, J.; Venclikova, K.; Jedelska, J.; Bakowsky, U.; Bosakova, Z.; Lhotka, M.; Vaculin, S.; et al. Poly(d,l-lactide)/polyethylene glycol micro/nanofiber mats as paclitaxel-eluting carriers: Preparation and characterization of fibers, in vitro drug release, antiangiogenic activity and tumor recurrence prevention. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 982–993. [Google Scholar] [CrossRef]
- Wulf, K.; Teske, M.; Matschegewski, C.; Arbeiter, D.; Bajer, D.; Eickner, T.; Schmitz, K.-P.; Grabow, N. Novel approach for a PTX/VEGF dual drug delivery system in cardiovascular applications-an innovative bulk and surface drug immobilization. Drug Deliv. Transl. Res. 2018, 8, 719–728. [Google Scholar] [CrossRef]
- Seidlitz, A.; Schick, W.; Reske, T.; Senz, V.; Grabow, N.; Petersen, S.; Nagel, S.; Weitschies, W. In vitro study of sirolimus release from a drug-eluting stent: Comparison of the release profiles obtained using different test setups. Eur. J. Pharm. Biopharm. 2015, 93, 328–338. [Google Scholar] [CrossRef] [PubMed]
- Venkatraman, S.; Boey, F. Release profiles in drug-eluting stents: Issues and uncertainties. J. Control Release 2007, 120, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Petersen, S.; Hussner, J.; Reske, T.; Grabow, N.; Senz, V.; Begunk, R.; Arbeiter, D.; Kroemer, H.K.; Schmitz, K.-P.; Meyer zu Schwabedissen, H.E.; et al. In vitro study of dual drug-eluting stents with locally focused sirolimus and atorvastatin release. J. Mater. Sci. Mater. Med. 2013, 24, 2589–2600. [Google Scholar] [CrossRef] [PubMed]
- Grabow, N.; Bünger, C.M.; Kischkel, S.; Timmermann, J.H.; Reske, T.; Martin, D.P.; Williams, S.F.; Schareck, W.; Sternberg, K.; Schmitz, K.-P. Development of a sirolimus-eluting poly (L-lactide)/poly(4-hydroxybutyrate) absorbable stent for peripheral vascular intervention. Biomed. Tech. (Berl.) 2013, 58, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Lincoff, A.M.; Furst, J.G.; Ellis, S.G.; Tuch, R.J.; Topol, E.J. Sustained Local Delivery of Dexamethasone by a Novel Intravascular Eluting Stent to Prevent Restenosis in the Porcine Coronary Injury Model. J. Am. Coll. Cardiol. 1997, 29, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Wissgott, C.; Schmidt, W.; Brandt, C.; Behrens, P.; Andresen, R. Preliminary Clinical Results and Mechanical Behavior of a New Double-Layer Carotid Stent. J. Endovasc. Ther. 2015, 22, 634–639. [Google Scholar] [CrossRef]
- Cardiovascular Implants—Endovascular Devices. Part 2—Vascular Stents; DIN EN ISO 25539-2:2019-07; Beuth Verlag GmbH: Berlin, Germany, 2019.
- Wu, D.; Zhang, Y.; Yuan, L.; Zhang, M.; Zhou, W. Viscoelastic interfacial properties of compatibilized poly(ε-caprolactone)/polylactide blend. J. Polym. Sci. B Polym. Phys. 2010, 48, 756–765. [Google Scholar] [CrossRef]
- Hilianen-Vainio, M.; Varpomaa, P. Modification of poly(L-lactides) by blending: Mechanical and hydrolytic behavior. Macromol. Chem. Phys. 1995, 197, 1503–1523. [Google Scholar] [CrossRef]
- López-Rodríguez, N.; López-Arraiza, A.; Meaurio, E.; Sarasua, J.R. Crystallization, morphology, and mechanical behavior of polylactide/poly(ε-caprolactone) blends. Polym. Eng. Sci. 2006, 46, 1299–1308. [Google Scholar] [CrossRef]
- Arbeiter, D.; Eickner, T.; Oschatz, S.; Reske, T.; Specht, O.; Teske, M.; Senz, V.; Schmitz, K.-P.; Grabow, N. Physico chemical and phase separation characterization of high molecular PLLA blended with low molecular PCL obtained from solvent cast processes. Mater. Res. Express 2020, 7, 95302. [Google Scholar] [CrossRef]
- Darie-Niţă, R.N.; Vasile, C.; Irimia, A.; Lipşa, R.; Râpă, M. Evaluation of some eco-friendly plasticizers for PLA films processing. J. Appl. Polym. Sci. 2016, 133. [Google Scholar] [CrossRef]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, S.; Fritz, H.G. Plasticizing polylactide?the effect of different plasticizers on the mechanical properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Baiardo, M.; Frisoni, G.; Scandola, M.; Rimelen, M.; Lips, D.; Ruffieux, K.; Wintermantel, E. Thermal and mechanical properties of plasticized poly(L-lactic acid). J. Appl. Polym. Sci. 2003, 90, 1731–1738. [Google Scholar] [CrossRef]
- Ozkoc, G.; Kemaloglu, S. Morphology, biodegradability, mechanical, and thermal properties of nanocomposite films based on PLA and plasticized PLA. J. Appl. Polym. Sci. 2009, 114, 2481–2487. [Google Scholar] [CrossRef]
- Ljungberg, N.; Wesslén, B. The effects of plasticizers on the dynamic mechanical and thermal properties of poly(lactic acid). J. Appl. Polym. Sci. 2002, 86, 1227–1234. [Google Scholar] [CrossRef]
- Siepmann, F.; Le Brun, V.; Siepmann, J. Drugs acting as plasticizers in polymeric systems: A quantitative treatment. J. Control Release 2006, 115, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, D.D.; Luo, L.-J.; Lai, J.-Y. Effects of shell thickness of hollow poly(lactic acid) nanoparticles on sustained drug delivery for pharmacological treatment of glaucoma. Acta Biomater. 2020, 302–315. [Google Scholar] [CrossRef]
- Lee, C.-H.; Li, Y.-J.; Huang, C.-C.; Lai, J.-Y. Poly(ε-caprolactone) nanocapsule carriers with sustained drug release: Single dose for long-term glaucoma treatment. Nanoscale 2017, 9, 11754–11764. [Google Scholar] [CrossRef]
- Wulf, K.; Arbeiter, D.; Matschegewski, C.; Teske, M.; Huling, J.; Schmitz, K.-P.; Grabow, N.; Kohse, S. Smart releasing electrospun nanofibers—Poly-L-lactide fibers as dual drug delivery system for biomedical application. Biomed. Mater. 2020. [Google Scholar] [CrossRef]
- Kunze, C.; Freier, T.; Kramer, S.; Schmitz, K.-P. Anti-inflammatory prodrugs as plasticizers for biodegradable implant materials based on poly(3-hydroxybutyrate). J. Mater. Sci. Mater. Med. 2002, 13, 1051–1055. [Google Scholar] [CrossRef] [PubMed]
- Savla, R.; Browne, J.; Plassat, V.; Wasan, K.M.; Wasan, E.K. Review and analysis of FDA approved drugs using lipid-based formulations. Drug Dev. Ind. Pharm. 2017, 43, 1743–1758. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Leo, A.; Hoekman, D. Exploring QSAR: Hydrophobic, Electronic, and Steric Constants. J. Med. Chem. 1996, 39, 1189–1190. [Google Scholar] [CrossRef]
- Lallemand, F.; Schmitt, M.; Bourges, J.-L.; Gurny, R.; Benita, S.; Garrigue, J.-S. Cyclosporine A delivery to the eye: A comprehensive review of academic and industrial efforts. Eur. J. Pharm. Biopharm. 2017, 117, 14–28. [Google Scholar] [CrossRef] [PubMed]
- Ihlenfeldt, W.D.; Takahashi, Y.; Abe, H.; Sasaki, S. Computation and management of chemical properties in CACTVS: An extensible networked approach toward modularity and compatibility. J. Chem. Inf. Model. 1994, 34, 109–116. [Google Scholar] [CrossRef]
- Tarini, M.; Cignoni, P.; Montani, C. Ambient occlusion and edge cueing to enhance real time molecular visualization. IEEE Trans. Vis. Comput. Graph. 2006, 12, 1237–1244. [Google Scholar] [CrossRef]
- Finkelstein, A.; McClean, D.; Kar, S.; Takizawa, K.; Varghese, K.; Baek, N.; Park, K.; Fishbein, M.C.; Makkar, R.; Litvack, F.; et al. Local drug delivery via a coronary stent with programmable release pharmacokinetics. Circulation 2003, 107, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Mochizuki, A.; Niikawa, T.; Omura, I.; Yamashita, S. Controlled release of argatroban from PLA film—Effect of hydroxylesters as additives on enhancement of drug release. J. Appl. Polym. Sci. 2008, 108, 3353–3360. [Google Scholar] [CrossRef]
- Shi, W.; Gu, C.; Jiang, H.; Zhang, M.; Lang, M. Effects of amphiphilic chitosan-g-poly(ε-caprolactone) polymer additives on paclitaxel release from drug eluting implants. Mater. Sci. Eng. C Mater. Biol. Appl. 2014, 45, 502–509. [Google Scholar] [CrossRef]
- Kim, S.M.; Park, S.-B.; Bedair, T.M.; Kim, M.-H.; Park, B.J.; Joung, Y.K.; Han, D.K. The effect of solvents and hydrophilic additive on stable coating and controllable sirolimus release system for drug-eluting stent. Mater. Sci. Eng. C Mater. Biol. Appl. 2017, 78, 39–46. [Google Scholar] [CrossRef]
- Livingston, M.; Tan, A. Coating Techniques and Release Kinetics of Drug-Eluting Stents. J. Med. Devices 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the structure of solution grown crystals of lactide copolymers by means of chemical reactions. Kolloid-Z. Z. Polym. 1973, 251, 980–990. [Google Scholar] [CrossRef]
- International Standard Organisation. Plastics-Determination of Tensile Properties; International Standard Organisation: Geneva, Switzerland, 2012. [Google Scholar]
- Migliaresi, C.; de Lollis, A.; Fambri, L.; Cohn, D. The effect of thermal history on the crystallinity of different molecular weight PLLA biodegradable polymers. Clin. Mater. 1991, 8, 111–118. [Google Scholar] [CrossRef]
- Freier, T.; Kunze, C.; Schmitz, K.-P. Solvent removal from solution-cast films of biodegradable polymers. J. Mate. Sci. Lett. 2001, 20, 1929–1931. [Google Scholar] [CrossRef]
- Liggins, R.T.; Burt, H.M. Paclitaxel loaded poly(L-lactic acid) (PLLA) microspheres. II. The effect of processing parameters on microsphere morphology and drug release kinetics. Int. J. Pharm. 2004, 281, 103–106. [Google Scholar] [CrossRef] [PubMed]
- Mandelkern, L. The crystallization kinetics of polymer-diluent mixtures: The temperature coefficient of the process. Polymer 1964, 5, 637–648. [Google Scholar] [CrossRef]
- Liggins, R.T.; Burt, H.M. Paclitaxel-loaded poly(L-lactic acid) microspheres 3: Blending low and high molecular weight polymers to control morphology and drug release. Int. J. Pharm. 2004, 282, 61–71. [Google Scholar] [CrossRef]
- Nukala, R.K.; Boyapally, H.; Slipper, I.J.; Mendham, A.P.; Douroumis, D. The application of electrostatic dry powder deposition technology to coat drug-eluting stents. Pharm. Res. 2010, 27, 72–81. [Google Scholar] [CrossRef]
- Martins, A.; Duarte, A.R.C.; Faria, S.; Marques, A.P.; Reis, R.L.; Neves, N.M. Osteogenic induction of hBMSCs by electrospun scaffolds with dexamethasone release functionality. Biomaterials 2010, 31, 5875–5885. [Google Scholar] [CrossRef] [Green Version]









| Stent | Stent Material | Polymer | Absorption Time | Drug | Drug-Eluting Time | Manufacturer | Drug Loading |
|---|---|---|---|---|---|---|---|
| Cypher | Stainless steel | PEVA/PBMA | Permanent | Sirolimus | 90 days | Cordis | 140 μg/cm2 |
| Taxus Express | Stainless steel | SIBS | Permanent | Paclitaxel | >180 days | Boston Scientific | 100 μg/cm2 |
| Xience Alpine | CoCr | PVDF- HFP | Permanent | Everolimus | 120 days | Abbott Laboratories | 100 μg/cm2 |
| Resolute Integrity | CoNi with Pt-Ir | BioLinx | Permanent | Zotarolimus | 180 days | Medtronic | - |
| Orsiro | CoCr | PLLA | 15 months | Sirolimus | 100–120 days | Biotronik | 1.4 μg/mm2 |
| Ultimaster | CoCr | PDLLA-PCL | 3–4 months | Sirolimus | 3–4 months | Terumo Interventional Systems | - |
| Synergy | PtCr | PLGA | 3–4 months | Everolimus | 3 months | Boston Scientific | 38–179 μg/stent |
| Nobori | Stainless steel | PDLLA | 6–9 months | Biolimus | 6–9 months | Terumo | 15.6 μg/mm2 |
| Drug | Structural Formula | Space Filling Model Illustration | PSA (in Å2) 1 | logP | MW (g/mol) |
|---|---|---|---|---|---|
| Paclitaxel (PTX) |  |  | 221 | 3 [18] | 853.9 |
| Sirolimus (SIR) |  |  | 195 | 7.45 [55] | 914.2 |
| Dexamethasone (DEX) |  |  | 95 | 1.83 [56] | 392.5 |
| CYCLOsporine (CYCLO) |  |  | 279 | 1.4 [57] | 1202.6 |
| PLLA/PTX | Tg (°C) | ΔHPLLA (J/g) | χ (%) | Tm,PLLA (°C) | ΔHPTX (J/g) | Tm,PTX (°C) |
| 100/0 | 72.8 ± 0.5 | 35.4 ± 1.8 | 37.8 ± 1.9 | 177.08 ± 0.25 | --- | --- |
| 90/10 | 75.4 ± 0.4 | 31 ± 7 | 33 ± 7 | 174.72 ± 0.19 | --- | --- |
| 85/15 | 74.4 ± 2.6 | 26 ± 10 | 27 ± 10 | 173.84 ± 0.07 | --- | --- |
| 80/20 | 69.2 ± 0.8 | 10.6 ± 1.6 | 11.3 ± 1.7 | 173.78 ± 0.13 | --- | --- |
| 0/100 | --- | --- | --- | --- | 32.4 ± 1.2 | 213.48 ± 0.17 |
| PLLA/SIR | Tg (°C) | ΔHPLLA (J/g) | χ (%) | Tm,PLLA (°C) | ΔHSIR (J/g) | Tm,SIR (°C) |
| 100/0 | 72.8 ± 0.5 | 35.4 ± 1.8 | 37.8 ± 1.9 | 177.08 ± 0.25 | --- | --- |
| 90/10 | 73.45 ± 0.24 | 30 ± 5 | 32 ± 6 | 175.5 ± 0.5 | 43 ± 5 | 199.5 ± 0.6 |
| 85/15 | 74.9 ± 0.7 | 38 ± 8 | 41 ± 9 | 174.82 ± 0.28 | 45 ± 13 | 198.6 ± 0.6 |
| 80/20 | 73.9 ± 0.5 | 48.5 ± 2.9 | 52 ± 3 | 174.32 ± 0.07 | 49 ± 13 | 196.5 ± 0.5 |
| 0/100 | --- | --- | --- | --- | 64.9 ± 2.8 | 183.9 ± 0.5 |
| PLLA/DEX | Tg (°C) | ΔHPLLA (J/g) | χ (%) | Tm,PLLA (°C) | ΔHDEX (J/g) | Tm,DEX* (°C) |
| 100/0 | 72.8 ± 0.5 | 35.4 ± 1.8 | 37.8 ± 1.9 | 177.08 ± 0.25 | --- | --- |
| 90/10 | 74.3 ± 0.5 | 44.0 ± 2.4 | 46.9 ± 2.6 | 177.3 ± 0.3 | 1.9 ± 0.8 | 231.8 ± 1.5 |
| 85/15 | 71.7 ± 1.7 | 38 ± 6 | 40 ± 6 | 177.0 ± 0.5 | 19 ± 4 | 238.2 ± 0.9 |
| 80/20 | 74.9 ± 0.5 | 42 ± 10 | 45 ± 11 | 177.69 ± 0.15 | 8 ± 5 | 240.1 ± 1.4 |
| 0/100 | --- | --- | --- | --- | 15.2 ± 2.8 | 263.5 ± 0.8 |
| PLLA/CYCLO | Tg (°C) | ΔHPLLA (J/g) | χ (%) | Tm,PLLA (°C) | ΔHCYCLO (J/g) | Tm,CYCLO (°C) |
| 100/0 | 72.8 ± 0.5 | 35.4 ± 1.8 | 37.8 ± 1.9 | 177.08 ± 0.25 | --- | --- |
| 90/10 | 74.5 ± 0.7 | 43.1 ± 2.8 | 46 ± 3 | 174.51 ± 0.21 | --- | --- |
| 85/15 | 75.9 ± 2.0 | 40 ± 4 | 42 ± 4 | 174.61 ± 0.02 | --- | --- |
| 80/20 | 72.7 ± 2.4 | 36 ± 5 | 38 ± 6 | 173.7 ± 0.7 | --- | --- |
| 0/100 | --- | --- | --- | --- | --- | 146.9 ± 0.7 |
| PLLA/PTX | E (MPa) | σmax (MPa) | εB (%) |
| 100/0 | 2250 ± 40 | 107.1 ± 1.3 | 9.9 ± 0.7 |
| 90/10 | 3020 ± 100 | 134 ± 4 | 23 ± 16 |
| 85/15 | 2980 ± 170 | 130 ± 16 | 16.1 ± 1.2 |
| 80/20 | 3320 ± 120 | 140 ± 5 | 12.3 ± 1.1 |
| PLLA/SIR | E (MPa) | σmax (MPa) | εB (%) |
| 100/0 | 2250 ± 40 | 107.1 ± 1.3 | 9.9 ± 0.7 |
| 90/10 | 3420 ± 220 | 156 ± 16 | 7.40 ± 0.17 |
| 85/15 | 3200 ± 700 | 160 ± 30 | 8.0 ± 0.7 |
| 80/20 | 2000 ± 220 | 115 ± 30 | 8.9 ± 0.8 |
| PLLA/DEX | E (MPa) | σmax (MPa) | εB (%) |
| 100/0 | 2250 ± 40 | 107.1 ± 1.3 | 9.9 ± 0.7 |
| 90/10 | 3000 ± 200 | 141 ± 9 | 8.8 ± 2.0 |
| 85/15 | 2940 ± 130 | 118 ± 8 | 11 ± 3 |
| 80/20 | 2690 ± 110 | 115 ± 2.4 | 6.1 ± 0.9 |
| PLLA/CYCLO | E (MPa) | σmax (MPa) | εB (%) |
| 100/0 | 2250 ± 40 | 107.1 ± 1.3 | 9.9 ± 0.7 |
| 90/10 | 2580 ± 240 | 119.2 ± 2.0 | 11 ± 6 |
| 85/15 | 2560 ± 110 | 107 ± 6 | 5.4 ± 0.7 |
| 80/20 | 2870 ± 190 | 141 ± 5 | 6.4 ± 1.7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arbeiter, D.; Reske, T.; Teske, M.; Bajer, D.; Senz, V.; Schmitz, K.-P.; Grabow, N.; Oschatz, S. Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices—A Systematic Study. Polymers 2021, 13, 292. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020292
Arbeiter D, Reske T, Teske M, Bajer D, Senz V, Schmitz K-P, Grabow N, Oschatz S. Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices—A Systematic Study. Polymers. 2021; 13(2):292. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020292
Chicago/Turabian StyleArbeiter, Daniela, Thomas Reske, Michael Teske, Dalibor Bajer, Volkmar Senz, Klaus-Peter Schmitz, Niels Grabow, and Stefan Oschatz. 2021. "Influence of Drug Incorporation on the Physico-Chemical Properties of Poly(l-Lactide) Implant Coating Matrices—A Systematic Study" Polymers 13, no. 2: 292. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020292