Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
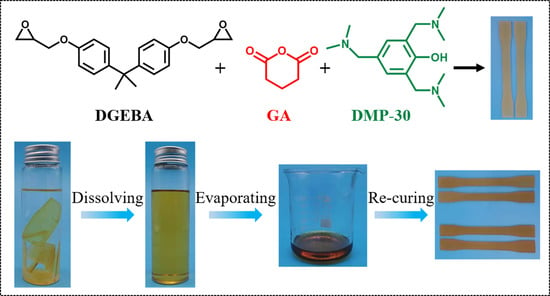
2.2. Preparation of Epoxy Resins
2.3. Characterizations
2.3.1. Differential Scanning Calorimeter (DSC)
2.3.2. Fourier Transform Infrared Spectroscopy (FTIR)
2.3.3. Dynamic Mechanical Analysis (DMA)
2.3.4. Uniaxial Tension Tests
2.3.5. Thermogravimetric Analysis (TGA)
2.4. Swelling and Dissolution Tests
2.5. Reuse of Decomposed Epoxy Oligomer
3. Results and Discussion
3.1. Preparation and Structural Characterization of Fresh Epoxy Resins
3.2. Thermal and Mechanical Properties of Epoxy Resins
3.3. Swelling and Dissolution of Epoxy Resins
3.4. Reuse of Decomposed Epoxy Oligomer
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vidil, T.; Tournilhac, F.; Musso, S.; Robisson, A.; Leibler, L. Control of reactions and network structures of epoxy thermosets. Prog. Polym. Sci. 2016, 62, 126–179. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Meng, L.-H.; Huang, Y.; Du, J. Recycling of carbon/epoxy composites. J. Appl. Polym. Sci. 2004, 94, 1912–1916. [Google Scholar] [CrossRef]
- Wang, B.-C.; Zhou, X.; Ma, K.-M. Fabrication and properties of CNTs/carbon fabric hybrid multiscale composites processed via resin transfer molding technique. Compos. Part B Eng. 2013, 46, 123–129. [Google Scholar] [CrossRef]
- Kuang, T.; Ju, J.; Yang, Z.; Geng, L.; Peng, X. A facile approach towards fabrication of lightweight biodegradable poly (butylene succinate)/carbon fiber composite foams with high electrical conductivity and strength. Compos. Sci. Technol. 2018, 159, 171–179. [Google Scholar] [CrossRef]
- Barra, G.; Guadagno, L.; Vertuccio, L.; Simonet, B.; Santos, B.; Zarrelli, M.; Arena, M.; Viscardi, M. Different methods of dispersing carbon nanotubes in epoxy resin and initial evaluation of the obtained nanocomposite as a matrix of carbon fiber reinforced laminate in terms of vibroacoustic performance and flammability. Materials 2019, 12, 2998. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, X.; Luo, C.; Li, Z.; Yu, K. Primary recycling of anhydride-cured engineering epoxy using alcohol solvent. Polym. Eng. Sci. 2019, 59, E111–E119. [Google Scholar] [CrossRef]
- Pickering, S.J. Recycling technologies for thermoset composite materials—current status. Compos. Part A Appl. Sci. Manuf. 2006, 37, 1206–1215. [Google Scholar] [CrossRef]
- Di Mauro, C.; Tran, T.-N.; Graillot, A.; Mija, A. Enhancing the recyclability of a vegetable oil-based epoxy thermoset through initiator influence. ACS Sustain. Chem. Eng. 2020, 8, 7690–7700. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Recyclable, repairable, and reshapable (3R) Thermoset materials with shape memory properties from bio-based epoxidized vegetable oils. ACS Appl. Bio Mater. 2020, 3, 8094–8104. [Google Scholar] [CrossRef]
- Dang, W.; Kubouchi, M.; Yamamoto, S.; Sembokuya, H.; Tsuda, K. An approach to chemical recycling of epoxy resin cured with amine using nitric acid. Polymer 2002, 43, 2953–2958. [Google Scholar] [CrossRef]
- Yang, P.; Zhou, Q.; Yuan, X.-X.; Van Kasteren, J.M.; Wang, Y.-Z. Highly efficient solvolysis of epoxy resin using poly(ethylene glycol)/NaOH systems. Polym. Degrad. Stab. 2012, 97, 1101–1106. [Google Scholar] [CrossRef]
- Piñero-Hernanz, R.; García-Serna, J.; Dodds, C.; Hyde, J.; Poliakoff, M.; Cocero, M.J.; Kingman, S.; Pickering, S.; Lester, E. Chemical recycling of carbon fibre composites using alcohols under subcritical and supercritical conditions. J. Supercrit. Fluids 2008, 46, 83–92. [Google Scholar] [CrossRef]
- Sekula, R.; Leszczynski, S. Utilization of scrap thermosets using pyrolysis. Energy Sources Part A Recovery Util. Environ. Eff. 2008, 31, 76–85. [Google Scholar] [CrossRef]
- Yuyan, L.; Guohua, S.; Linghui, M. Recycling of carbon fibre reinforced composites using water in subcritical conditions. Mater. Sci. Eng. A 2009, 520, 179–183. [Google Scholar] [CrossRef]
- Scheutz, G.M.; Lessard, J.J.; Sims, M.B.; Sumerlin, B.S. Adaptable Crosslinks in polymeric materials: resolving the intersection of thermoplastics and thermosets. J. Am. Chem. Soc. 2019, 141, 16181–16196. [Google Scholar] [CrossRef]
- Guerre, M.; Taplan, C.; Winne, J.M.; Du Prez, F.E. Vitrimers: DIRECTING chemical reactivity to control material properties. Chem. Sci. 2020, 11, 4855–4870. [Google Scholar] [CrossRef] [Green Version]
- Hayashi, M. Implantation of recyclability and healability into cross-linked commercial polymers by applying the vitrimer concept. Polymers 2020, 12, 1322. [Google Scholar] [CrossRef]
- Liu, T.; Zhao, B.; Zhang, J. Recent development of repairable, malleable and recyclable thermosetting polymers through dynamic transesterification. Polymers 2020, 194. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, Y.; Ji, Y.; Wei, Y. Functional epoxy vitrimers and composites. Prog. Mater. Sci. 2020, 25, 100710. [Google Scholar] [CrossRef]
- Montarnal, D.; Capelot, M.; Tournilhac, F.; Leibler, L. Silica-like malleable materials from permanent organic networks. Science 2011, 334, 965–968. [Google Scholar] [CrossRef]
- Chen, L.; Zhu, S.; Toendepi, I.; Jiang, Q.; Wei, Y.; Qiu, Y.; Liu, W. Reprocessable, reworkable, and mechanochromic polyhexahydrotriazine thermoset with multiple stimulus responsiveness. Polymers 2020, 12, 2375. [Google Scholar] [CrossRef] [PubMed]
- Hamel, C.M.; Kuang, X.; Chen, K.; Qi, H.J. Reaction-diffusion model for thermosetting polymer dissolution through exchange reactions assisted by small-molecule solvents. Macromolecules 2019, 52, 3636–3645. [Google Scholar] [CrossRef]
- Di Mauro, C.; Malburet, S.; Genua, A.; Graillot, A.; Mija, A. Sustainable series of new epoxidized vegetable oils-based thermosets with chemical recycling properties. Biomacromolecules 2020, 21, 3923–3935. [Google Scholar] [CrossRef]
- Di Mauro, C.; Genua, A.; Mija, A. Building thermally and chemically reversible covalent bonds in vegetable oil based epoxy thermosets. Influence of epoxy–hardener ratio in promoting recyclability. Mater. Adv. 2020, 1, 1788–1798. [Google Scholar] [CrossRef]
- Tran, T.-N.; Di Mauro, C.; Malburet, S.; Graillot, A.; Mija, A. Dual cross-linking of epoxidized linseed oil with combined aliphatic/aromatic diacids containing dynamic s−s bonds generating recyclable thermosets. ACS Appl. Bio Mater. 2020, 3, 7550–7561. [Google Scholar] [CrossRef]
- De Luzuriaga, A.R.; Martin, R.; Markaide, N.; Rekondo, A.; Cabañero, G.; Rodríguez, J.; Odriozola, I. Epoxy resin with exchangeable disulfide crosslinks to obtain reprocessable, repairable and recyclable fiber-reinforced thermoset composites. Mater. Horiz. 2016, 3, 241–247. [Google Scholar] [CrossRef]
- Ciaccia, M.; Cacciapaglia, R.; Mencarelli, P.; Mandolini, L.; Di Stefano, S. Fast transimination in organic solvents in the absence of proton and metal catalysts. A key to imine metathesis catalyzed by primary amines under mild conditions. Chem. Sci. 2013, 4, 2253–2261. [Google Scholar] [CrossRef]
- Ying, H.; Zhang, Y.; Cheng, J. Dynamic urea bond for the design of reversible and self-healing polymers. Nat. Commun. 2014, 5, 1–9. [Google Scholar] [CrossRef]
- Capelot, M.; Montarnal, D.; Tournilhac, F.; Leibler, L. Metal-catalyzed transesterification for healing and assembling of thermosets. J. Am. Chem. Soc. 2012, 134, 7664–7667. [Google Scholar] [CrossRef]
- Lu, L.; Pan, J.; Li, G. Recyclable high-performance epoxy based on transesterification reaction. J. Mater. Chem. A 2017, 5, 21505–21513. [Google Scholar] [CrossRef]
- Yue, L.; Guo, H.; Kennedy, A.; Patel, A.; Gong, X.; Ju, T.; Gray, T.; Manas-Zloczower, I. vitrimerization: converting thermoset polymers into vitrimers. ACS Macro Lett. 2020, 9, 836–842. [Google Scholar] [CrossRef]
- Shi, Q.; Yu, K.; Dunn, M.L.; Wang, T.; Qi, H.J. Solvent assisted pressure-free surface welding and reprocessing of malleable epoxy polymers. Macromolecules 2016, 49, 5527–5537. [Google Scholar] [CrossRef]
- Zhang, S.; Liu, T.; Hao, C.; Wang, L.; Han, J.; Liu, H.; Zhang, J. Preparation of a lignin-based vitrimer material and its potential use for recoverable adhesives. Green Chem. 2018, 20, 2995–3000. [Google Scholar] [CrossRef]
- Yu, K.; Shi, Q.; Dunn, M.L.; Wang, T.; Qi, H.J. Carbon fiber reinforced thermoset composite with near 100% recyclability. Adv. Funct. Mater. 2016, 26, 6098–6106. [Google Scholar] [CrossRef]
- Chabert, E.; Vial, J.; Cauchois, J.-P.; Mihaluta, M.; Tournilhac, F. Multiple welding of long fiber epoxy vitrimer composites. Soft Matter 2016, 12, 4838–4845. [Google Scholar] [CrossRef] [PubMed]
- Yu, K.; Shi, Q.; Li, H.; Jabour, J.; Yang, H.; Dunn, M.L.; Wang, T.; Qi, H.J. Interfacial welding of dynamic covalent network polymers. J. Mech. Phys. Solids 2016, 94, 1–17. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Reprocessing and recycling of thermosetting polymers based on bond exchange reactions. RSC Adv. 2014, 4, 10108–10117. [Google Scholar] [CrossRef]
- Zhang, B.; Yuan, C.; Zhang, W.; Dunn, M.L.; Qi, H.J.; Liu, Z.; Yu, K.; Ge, Q. Recycling of vitrimer blends with tunable thermomechanical properties. RSC Adv. 2019, 9, 5431–5437. [Google Scholar] [CrossRef] [Green Version]
- Yu, K.; Yang, H.; Dao, B.H.; Shi, Q.; Yakacki, C.M. Dissolution of covalent adaptable network polymers in organic solvent. J. Mech. Phys. Solids 2017, 109, 78–94. [Google Scholar] [CrossRef]
- Kuang, X.; Shi, Q.; Zhou, Y.; Zhao, Z.; Wang, T.; Qi, H.J. Dissolution of epoxy thermosets via mild alcoholysis: The mechanism and kinetics study. RSC Adv. 2018, 8, 1493–1502. [Google Scholar] [CrossRef] [Green Version]
- Kuang, X.; Zhou, Y.; Shi, Q.; Wang, T.; Qi, H.J. Recycling of epoxy thermoset and composites via good solvent assisted and small molecules participated exchange reactions. ACS Sustain. Chem. Eng. 2018, 6, 9189–9197. [Google Scholar] [CrossRef]
- Demongeot, A.; Groote, R.; Goossens, H.; Hoeks, T.; Tournilhac, F.; Leibler, L. Cross-linking of Poly(butylene terephthalate) by reactive extrusion using Zn(II) epoxy-vitrimer chemistry. Macromolecules 2017, 50, 6117–6127. [Google Scholar] [CrossRef] [Green Version]
- Brutman, J.P.; Delgado, P.A.; Hillmyer, M.A. Polylactide vitrimers. ACS Macro Lett. 2014, 3, 607–610. [Google Scholar] [CrossRef] [Green Version]
- Capelot, M.; Unterlass, M.M.; Tournilhac, F.; Leibler, L. Catalytic control of the vitrimer glass transition. ACS Macro Lett. 2012, 1, 789–792. [Google Scholar] [CrossRef]
- Tan, J.; Li, C.; De Bruycker, K.; Zhang, G.; Gu, J.; Zhang, Q. Recyclable cross-linked hydroxythioether particles with tunable structures via robust and efficient thiol-epoxy dispersion polymerizations. RSC Adv. 2017, 7, 51763–51772. [Google Scholar] [CrossRef] [Green Version]
- Altuna, F.I.; Hoppe, C.E.; Williams, R.J. Epoxy vitrimers with a covalently bonded tertiary amine as catalyst of the transesterification reaction. Eur. Polym. J. 2019, 113, 297–304. [Google Scholar] [CrossRef]
- Liu, T.; Zhang, S.; Hao, C.; Verdi, C.; Liu, W.; Liu, H.; Zhang, J. Glycerol induced catalyst-free curing of epoxy and vitrimer preparation. Macromol. Rapid Commun. 2019, 40, e1800889. [Google Scholar] [CrossRef]
- Delahaye, M.; Winne, J.M.; Du Prez, F.E. Internal catalysis in covalent adaptable networks: phthalate monoester transesterification as a versatile dynamic cross-linking chemistry. J. Am. Chem. Soc. 2019, 141, 15277–15287. [Google Scholar] [CrossRef]
- Han, J.; Liu, T.; Hao, C.; Zhang, S.; Guo, B.; Zhang, J. A Catalyst-free epoxy vitrimer system based on multifunctional hyperbranched polymer. Macromolecules 2018, 51, 6789–6799. [Google Scholar] [CrossRef]
- Li, Y.; Liu, T.; Zhang, S.; Shao, L.; Fei, M.; Yu, H.; Zhang, J. Catalyst-free vitrimer elastomers based on a dimer acid: Robust mechanical performance, adaptability and hydrothermal recyclability. Green Chem. 2020, 22, 870–881. [Google Scholar] [CrossRef]
- Hao, C.; Liu, T.; Zhang, S.; Liu, W.; Shan, Y.; Zhang, J. Triethanolamine-mediated covalent adaptable epoxy network: excellent mechanical properties, fast repairing, and easy recycling. Macromolecules 2020, 53, 3110–3118. [Google Scholar] [CrossRef]
- Yang, T.; Zhang, C.; Zhang, J.; Cheng, J. The influence of tertiary amine accelerators on the curing behaviors of epoxy/anhydride systems. Thermochim. Acta 2014, 577, 11–16. [Google Scholar] [CrossRef]
- Ellis, B. Curing agents for epoxy resins. In Chemistry and Technology of Epoxy Resins, 1st ed.; Ellies, B., Ed.; Springer: Berlin/Heidelberg, Germany, 1993; pp. 37–70. [Google Scholar]
- Montserrat, S.; Flaqué, C.; Pagès, P.; Málek, J. Effect of the crosslinking degree on curing kinetics of an epoxy–anhydride system. J. Appl. Polym. Sci. 1995, 56, 1413–1421. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kakiuchi, H. Study of epoxy compounds. Part I. Curing reactions of epoxy resin and acid anhydride with amine and alcohol as catalyst. J. Appl. Polym. Sci. 1963, 7, 1063–1081. [Google Scholar] [CrossRef]
- Tanaka, Y.; Kakiuchi, H. Study of epoxy compounds. Part VI. Curing reactions of epoxy resin and acid anhydride with amine, acid, alcohol, and phenol as catalysts. J. Polym. Sci. Part A Gen. Pap. 1964, 2, 3405–3430. [Google Scholar] [CrossRef]
- Antoon, M.K.; Koenig, J.L. Crosslinking mechanism of an anhydride-cured epoxy resin as studied by fourier transform infrared spectroscopy. J. Polym. Sci. Polym. Chem. Ed. 1981, 19, 549–570. [Google Scholar] [CrossRef]
- Wang, D.; Li, L.Y.; Ke, H.J.; Xu, K.L.; Lu, S.; Gong, W.H.; Zhang, H.; Wang, G.Y.; Zhao, Y.M.; Zhao, N. Preparation and properties of recyclable high-performance epoxy resins and composites. Acta Polym. Sin. 2020, 51, 303–310. [Google Scholar] [CrossRef]
- Liu, T.; Hao, C.; Wang, L.; Li, Y.; Liu, W.; Xin, J.; Zhang, J. Eugenol-derived biobased epoxy: shape memory, repairing, and recyclability. Macromolecules 2017, 50, 8588–8597. [Google Scholar] [CrossRef]
- Bellenger, V.; Verdu, J.; Morel, E. Effect of structure on glass transition temperature of amine crosslinked epoxies. J. Polym. Sci. Part B Polym. Phys. 1987, 25, 1219–1234. [Google Scholar] [CrossRef]
- Bellenger, V.; Morel, E.; Verdu, J. Structure-properties relationships for densely crosslinked epoxide-amine systems based on epoxide or amine mixtures. J. Mater. Sci. 1988, 23, 4244–4250. [Google Scholar] [CrossRef]
- Bellenger, V.; Verdu, J.; Morel, E. Structure-properties relationships for densely cross-linked epoxide-amine systems based on epoxide or amine mixtures, Part 2 Water Absorption and Diffusion. J. Mater. Sci. 1989, 24, 63–68. [Google Scholar] [CrossRef]
- Morel, E.; Bellenger, V.; Bocquet, M.; Verdu, J. Structure-properties relationships for densely cross-linked epoxide-amine systems based on epoxide or amine mixtures, Part 3 Elastic Properties. J. Mater. Sci. 1989, 24, 69–75. [Google Scholar] [CrossRef]
- Askadskii, A.A. Influence of crosslinking density on the properties of polymer networks. Polym. Sci. USSR 1990, 32, 2061–2069. [Google Scholar] [CrossRef]
- Guerrero, P.; De la Caba, K.; Valea, A.; Corcuera, M.A.; Mondragon, I. Influence of cure schedule and stoichiometry on the dynamic mechanical behaviour of tetrafunctional epoxy resins cured with anhydrides. Polymer 1996, 37, 2195–2200. [Google Scholar] [CrossRef]
- Fernandez-Nograro, F.; Valea, A.; Llano-Ponte, R.; Mondragon, I. Dynamic and mechanical properties of DGEBA/poly(propylene oxide) amine based epoxy resins as a function of stoichiometry. Eur. Polym. J. 1996, 32, 257–266. [Google Scholar] [CrossRef]
- Bellenger, V.; Dhaoui, W.; Verdu, J.; Boye, J.; Lacabanne, C. Internal antiplasticization in diglycidyl ether of bisphenol A diamino diphenyl methane non-stoichiometric epoxy networks. Polym. Eng. Sci. 1990, 30, 321–325. [Google Scholar] [CrossRef]
- Palmese, G.R.; McCullough, R.L. Effect of epoxy–amine stoichiometry on cured resin material properties. J. Appl. Polym. Sci. 1992, 46, 1863–1873. [Google Scholar] [CrossRef]
- Morgan, R.J.; Kong, F.-M.; Walkup, C.M. Structure-property relations of polyethertriamine-cured bisphenol-A-diglycidyl ether epoxies. Polymer 1984, 25, 375–386. [Google Scholar] [CrossRef]
- Yu, K.; Taynton, P.; Zhang, W.; Dunn, M.L.; Qi, H.J. Influence of stoichiometry on the glass transition and bond exchange reactions in epoxy thermoset polymers. RSC Adv. 2014, 4, 48682–48690. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Cheng, K. Effect of crosslink density on some properties of electron beam-irradiated styrene–butadiene rubber. Radiat. Phys. Chem. 2009, 78, 1001–1005. [Google Scholar] [CrossRef]
- Dyakonov, T.; Mann, P.J.; Chen, Y.; Stevenson, W.T.K. Thermal analysis of some aromatic amine cured model epoxy resin systems—II: Residues of degradation. Polym. Degrad. Stab. 1996, 54, 67–83. [Google Scholar] [CrossRef]
- Freedman, B.; Butterfield, R.O.; Pryde, E.H. Transesterification kinetics of soybean oil 1. J. Am. Oil Chem. Soc. 1986, 63, 1375–1380. [Google Scholar] [CrossRef]
- Dixit, V.; Nagpal, A.K.; Singhal, R. Influence of phenoxy modifier on mechanical, electrical and morphological properties of epoxy/DDS system. Int. J. Plast. Technol. 2010, 14, 38–52. [Google Scholar] [CrossRef]
- Yang, G.; Pan, Q.; Pan, W.; Fu, S. Influence of flexible amine modifier on mechanical properties of epoxy resins. J. Mater. Eng. 2006, 5, 16–24. [Google Scholar] [CrossRef]











| Name | r1 (mol:mol) | DMP-30 2 (wt%) |
|---|---|---|
| Epoxy1 | 1:1.00 | 2 |
| Epoxy2 | 1:1.25 | 2 |
| Epoxy3 | 1:1.50 | 2 |
| Epoxy4 | 1:1.75 | 2 |
| Epoxy5 | 1:2.00 | 2 |
| System | r | Tg (°C) | Rubbery Modulus (MPa) | Crosslink Density (mmol/cm3) | Elastic Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|---|
| Epoxy1 | 1:1.00 | 46.39 | 5.28 | 0.57 | 1155.05 ± 9.87 | 42.24 ± 1.89 | 5.16 ± 0.33 |
| Epoxy2 | 1:1.25 | 54.70 | 10.03 | 1.06 | 1373.79 ± 34.23 | 52.11 ± 1.80 | 5.62 ± 0.23 |
| Epoxy3 | 1:1.50 | 65.06 | 15.23 | 1.57 | 1390.24 ± 82.83 | 65.59 ± 2.11 | 9.22 ± 0.31 |
| Epoxy4 | 1:1.75 | 74.60 | 21.04 | 2.12 | 1342.37 ± 41.81 | 62.33 ± 0.63 | 9.93 ± 0.83 |
| Epoxy5 | 1:2.00 | 87.73 | 29.86 | 2.91 | 1168.54 ± 35.09 | 55.39 ± 0.52 | 11.15 ± 0.84 |
| System | r | T5%1 (°C) | T10%2 (°C) | Tmax3 (°C) |
|---|---|---|---|---|
| Epoxy1 | 1:1.00 | 357 | 390 | 427 |
| Epoxy2 | 1:1.25 | 377 | 398 | 427 |
| Epoxy3 | 1:1.50 | 383 | 401 | 426 |
| Epoxy4 | 1:1.75 | 384 | 401 | 423 |
| Epoxy5 | 1:2.00 | 386 | 401 | 425 |
| System | r | Organic Solution | Conditions | ms/m01 (%) | md/m02 (%) |
|---|---|---|---|---|---|
| Epoxy1 | 1:1.00 | DMF | 25 °C for 96 h | 181.3 | 95.9 |
| TCB | 103.1 | 99.9 | |||
| xylenes | 101.8 | 99.8 | |||
| THF | 170.5 | 95.3 | |||
| heptane | 100.7 | 100.0 | |||
| Epoxy1 | 1:1.00 | TCB | 180 °C for 12 h | 159.8 | 95.5 |
| Epoxy2 | 1:1.25 | 155.9 | 95.9 | ||
| Epoxy3 | 1:1.50 | 146.3 | 98.8 | ||
| Epoxy4 | 1:1.75 | 139.3 | 99.7 | ||
| Epoxy5 | 1:2.00 | 134.8 | 99.6 |
| System | Tg (°C) | Rubbery Modulus (MPa) | Crosslink Density (mmol/cm3) | Elastic Modulus (MPa) | Tensile Strength (MPa) | Elongation at Break (%) |
|---|---|---|---|---|---|---|
| Epoxy5 | 87.73 | 31.13 | 2.91 | 1168.54 ± 35.09 | 55.39 ± 0.52 | 11.15 ± 0.84 |
| REP5-10 1 | 67.10 | 20.48 | 2.10 | 1018.39 ± 25.73 | 46.88 ± 0.19 | 16.78 ± 1.67 |
| REP5-20 2 | 52.26 | 11.94 | 1.28 | 971.01 ± 2.59 | 36.48 ± 0.03 | 195.36 ± 14.97 |
| REP5-100 3 | 49.60 | 6.27 | 0.67 | 1008.26 ± 18.08 | 18.81 ± 2.12 | 101.79 ± 5.71 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, W.; An, L.; Wang, S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers 2021, 13, 296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020296
Zhao W, An L, Wang S. Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions. Polymers. 2021; 13(2):296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020296
Chicago/Turabian StyleZhao, Wenzhe, Le An, and Shujuan Wang. 2021. "Recyclable High-Performance Epoxy-Anhydride Resins with DMP-30 as the Catalyst of Transesterification Reactions" Polymers 13, no. 2: 296. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13020296