Recent Advancements in Microbial Polysaccharides: Synthesis and Applications
Abstract
:1. Introduction
2. Biosynthetic Pathways
3. Targeted Polysaccharides Structures Modifications with Molecular Biology Tools
3.1. Role of Genetics in Polymers Production Systems
3.2. Role of Genetics in Polysaccharides Over-Expression
3.2.1. Recombinant of Hyaluronic Acid (HA) and Alginate Production
3.2.2. Biopolymer Production: Intracellular vs. Extracellular
4. Cell Functions of Microbial Polysaccharides
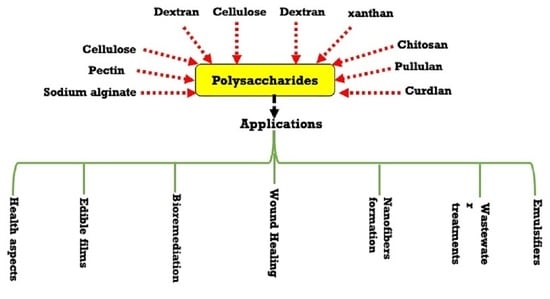
5. Applications of Different Polysaccharides
5.1. Food Applications of Different Polysaccharides
5.1.1. Cellulose
5.1.2. Xanthan
5.1.3. Dextran
5.1.4. Pullulan
5.2. Other Applications of Polysaccharides
5.2.1. Health Aspects
5.2.2. Medical Application
5.2.3. Emulsifiers
5.2.4. Polysaccharide-Based Edible Films
5.2.5. Wastewater and Bioremediation
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mudgil, D.; Barak, S. Composition, properties and health benefits of indigestible carbohydrate polymers as dietary fiber: A review. Int. J. Biol. Macromol. 2013, 61, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Khaskheli, S.G.; Zheng, W.; Sheikh, S.A.; Khaskheli, A.A.; Liu, Y.; Soomro, A.H.; Feng, X.; Sauer, M.B.; Wang, Y.F.; Huang, W. Characterization of Auricularia auricula polysaccharides and its antioxidant properties in fresh and pickled product. Int. J. Biol. Macromol. 2015, 81, 387–395. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, F.; Xu, Z.; Ding, Z. Bioactive mushroom polysaccharides: A review on monosaccharide composition, biosynthesis and regulation. Molecules 2017, 22, 955. [Google Scholar] [CrossRef] [Green Version]
- Ahmad, M.M. Characterization and antioxidant activities of polysaccharides extracted from flageolet bean pods waste. Curr. Res. Green Sustain. Chem. 2021, 4, 100154. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Sindhu, R.K.; Goyal, A.; Das, J.; Choden, S.; Kumar, P. Immunomodulatory potential of polysaccharides derived from plants and microbes: A narrative review. Carbohydr. Polym. Technol. Appl. 2021, 2, 100044. [Google Scholar] [CrossRef]
- Shakya, A.K. Unit-7 Polysaccharides; Indira Gandhi National Open University: New Delhi, India, 2020. [Google Scholar]
- Zheng, Y.; Xie, Q.; Wang, H.; Hu, Y.; Ren, B.; Li, X. Recent advances in plant polysaccharide-mediated nano drug delivery systems. Int. J. Biol. Macromol. 2020, 165, 2668–2683. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.R.; de Carvalho Junior, R.N. Polysaccharides obtained from natural edible sources and their role in modulating the immune system: Biologically active potential that can be exploited against COVID-19. Trends Food Sci. Technol. 2021, 108, 223–235. [Google Scholar] [CrossRef]
- Chaudhuri, S.; Datta, H.K. Macrofungal Polysaccharides as Immunoceuticals in Cancer Therapy. In Advances in Macrofungi; CRC Press: Boca Raton, FL, USA, 2021; pp. 287–310. ISBN 1003191274. [Google Scholar]
- Qu, J.; Huang, P.; Zhang, L.; Qiu, Y.; Qi, H.; Leng, A.; Shang, D. Hepatoprotective effect of plant polysaccharides from natural resources: A review of the mechanisms and structure-activity relationship. Int. J. Biol. Macromol. 2020, 161, 24–34. [Google Scholar] [CrossRef]
- Tahmouzi, S.; Ghodsi, M. Optimum extraction of polysaccharides from motherwort leaf and its antioxidant and antimicrobial activities. Carbohydr. Polym. 2014, 112, 396–403. [Google Scholar] [CrossRef]
- Tahmouzi, S. Optimization of polysaccharides from Zagros oak leaf using RSM: Antioxidant and antimicrobial activities. Carbohydr. Polym. 2014, 106, 238–246. [Google Scholar] [CrossRef]
- Santra, H.K.; Banerjee, D. Microbial Exopolysaccharides: Structure and Therapeutic Properties. In Microbial Polymers; Springer: Berlin/Heidelberg, Germany, 2021; pp. 375–420. [Google Scholar]
- Alsaadi, L.G.; Baker, B.A.A.; Kadhem, B.M.; Mahdi, L.H.; Mater, H.N. Exopolysaccharide as Antiviral, Antimicrobial And As Immunostimulants: A Review. Plant Arch. 2020, 20, 5859–5875. [Google Scholar]
- Günl, M.; Pauly, M. AXY3 encodes a α-xylosidase that impacts the structure and accessibility of the hemicellulose xyloglucan in Arabidopsis plant cell walls. Planta 2011, 233, 707–719. [Google Scholar] [CrossRef] [Green Version]
- Günl, M.; Neumetzler, L.; Kraemer, F.; de Souza, A.; Schultink, A.; Pena, M.; York, W.S.; Pauly, M. AXY8 encodes an α-fucosidase, underscoring the importance of apoplastic metabolism on the fine structure of Arabidopsis cell wall polysaccharides. Plant Cell 2011, 23, 4025–4040. [Google Scholar] [CrossRef] [Green Version]
- Gothwal, R.; Shashidhar, T. Antibiotic pollution in the environment: A review. Clean–Soil Air Water 2015, 43, 479–489. [Google Scholar] [CrossRef]
- Horton, D.; Wolfrom, M.L. Chapter VII: Section b: Polysaccharides:(Excluding Glycuronans, Bacterial Polysaccharides and Mucopolysaccharides). In Comprehensive Biochemistry; Elsevier: Amsterdam, The Netherlands, 1963; Volume 5, pp. 189–232. ISBN 0069-8032. [Google Scholar]
- Lapasin, R. Rheology of Industrial Polysaccharides: Theory and Applications; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012; ISBN 1461521858. [Google Scholar]
- Nicolaus, B.; Kambourova, M.; Oner, E.T. Exopolysaccharides from extremophiles: From fundamentals to biotechnology. Environ. Technol. 2010, 31, 1145–1158. [Google Scholar] [CrossRef]
- Jindal, N.; Khattar, J.S. Microbial polysaccharides in food industry. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 95–123. [Google Scholar]
- Ahmad, N.H.; Mustafa, S.; Che Man, Y.B. Microbial polysaccharides and their modification approaches: A review. Int. J. Food Prop. 2015, 18, 332–347. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.-M.; Kim, S.-W.; Hwang, H.-J.; Park, M.-K.; Mahmoud, Y.A.-G.; Choi, J.-W.; Yun, J.-W. Influence of agitation intensity and aeration rate on production of antioxidative exopolysaccharides from submerged mycelial culture of Ganoderma resinaceum. J. Microbiol. Biotechnol. 2006, 16, 1240–1247. [Google Scholar]
- Andrew, M.; Jayaraman, G. Structural features of microbial exopolysaccharides in relation to their antioxidant activity. Carbohydr. Res. 2020, 487, 107881. [Google Scholar] [CrossRef]
- Prameela, K.; Mohan, C.M.; Ramakrishna, C. Biopolymers for food design: Consumer-friendly natural ingredients. In Biopolymers for Food Design; Elsevier: Amsterdam, The Netherlands, 2018; pp. 1–3. [Google Scholar]
- Mangaraj, S.; Yadav, A.; Bal, L.M.; Dash, S.K.; Mahanti, N.K. Application of Biodegradable Polymers in Food Packaging Industry: A Comprehensive Review. J. Packag. Technol. Res. 2019, 3, 77–96. [Google Scholar] [CrossRef]
- Rehm, B.H.A.; Valla, S. Bacterial alginates: Biosynthesis and applications. Appl. Microbiol. Biotechnol. 1997, 48, 281–288. [Google Scholar] [CrossRef]
- Bakshi, P.S.; Selvakumar, D.; Kadirvelu, K.; Kumar, N.S. Chitosan as an environment friendly biomaterial—A review on recent modifications and applications. Int. J. Biol. Macromol. 2020, 150, 1072–1083. [Google Scholar] [CrossRef] [PubMed]
- Bose, S.; Koski, C.; Vu, A.A. Additive manufacturing of natural biopolymers and composites for bone tissue engineering. Mater. Horizons 2020, 7, 2011–2027. [Google Scholar] [CrossRef]
- Moradali, M.F.; Rehm, B.H.A. Bacterial biopolymers: From pathogenesis to advanced materials. Nat. Rev. Microbiol. 2020, 18, 195–210. [Google Scholar] [CrossRef] [PubMed]
- Steinbüchel, A. Perspectives for biotechnological production and utilization of biopolymers: Metabolic engineering of polyhydroxyalkanoate biosynthesis pathways as a successful example. Macromol. Biosci. 2001, 1, 1–24. [Google Scholar] [CrossRef]
- Pandey, S.; Shreshtha, I.; Sachan, S.G. Pullulan: Biosynthesis, Production and Applications. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer: Amsterdam, The Netherlands, 2021; pp. 121–141. [Google Scholar]
- Wang, J.; Salem, D.R.; Sani, R.K. Microbial polymers produced from methane: Overview of recent progress and new perspectives. In Microbial and Natural Macromolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 117–142. [Google Scholar]
- Salazar, N.; Gueimonde, M.; De Los Reyes-Gavilán, C.G.; Ruas-Madiedo, P. Exopolysaccharides produced by lactic acid bacteria and bifidobacteria as fermentable substrates by the intestinal microbiota. Crit. Rev. Food Sci. Nutr. 2016, 56, 1440–1453. [Google Scholar] [CrossRef]
- Tang, Y.; Dai, X.; Dong, B.; Guo, Y.; Dai, L. Humification in extracellular polymeric substances (EPS) dominates methane release and EPS reconstruction during the sludge stabilization of high-solid anaerobic digestion. Water Res. 2020, 175, 115686. [Google Scholar] [CrossRef]
- Sun, X.; Zhang, J. Bacterial exopolysaccharides: Chemical structures, gene clusters and genetic engineering. Int. J. Biol. Macromol. 2021, 173, 481–490. [Google Scholar] [CrossRef]
- Jiang, X.; Bai, J.; Yuan, J.; Zhang, H.; Lu, G.; Wang, Y.; Jiang, L.; Liu, B.; Wang, L.; Huang, D. High efficiency biosynthesis of O-polysaccharide-based vaccines against extraintestinal pathogenic Escherichia coli. Carbohydr. Polym. 2021, 255, 117475. [Google Scholar] [CrossRef]
- Hooper, A.B.; DiSpirito, A.A. In bacteria which grow on simple reductants, generation of a proton gradient involves extracytoplasmic oxidation of substrate. Microbiol. Rev. 1985, 49, 140–157. [Google Scholar] [CrossRef]
- More, T.T.; Yadav, J.S.S.; Yan, S.; Tyagi, R.D.; Surampalli, R.Y. Extracellular polymeric substances of bacteria and their potential environmental applications. J. Environ. Manag. 2014, 144, 1–25. [Google Scholar] [CrossRef]
- Mohd Nadzir, M.; Nurhayati, R.W.; Idris, F.N.; Nguyen, M.H. Biomedical applications of bacterial exopolysaccharides: A review. Polymers 2021, 13, 530. [Google Scholar] [CrossRef]
- Mandakovic, D.; Cintolesi, Á.; Maldonado, J.; Mendoza, S.N.; Aïte, M.; Gaete, A.; Saitua, F.; Allende, M.; Cambiazo, V.; Siegel, A. Genome-scale metabolic models of Microbacterium species isolated from a high altitude desert environment. Sci. Rep. 2020, 10, 5560. [Google Scholar] [CrossRef] [Green Version]
- Vaishnav, A.M.; Upadhyay, K.H.; Tipre, D.R.; Dave, S.R. Bio-prospecting of Fruits Waste for Exopolysaccharide Production by Bacteria. In Biotechnology for Sustainable Environment; Springer: Berlin/Heidelberg, Germany, 2021; pp. 353–371. [Google Scholar]
- Wiseman, B.; Nitharwal, R.G.; Widmalm, G.; Högbom, M. Structure of a full-length bacterial polysaccharide co-polymerase. Nat. Commun. 2021, 12, 369. [Google Scholar] [CrossRef]
- Soumya, M.P.; Nampoothiri, K.M. An overview of functional genomics and relevance of glycosyltransferases in exopolysaccharide production by lactic acid bacteria. Int. J. Biol. Macromol. 2021, 184, 1014–1025. [Google Scholar] [CrossRef]
- Tiwari, U.P.; Fleming, S.A.; Rasheed, M.S.A.; Jha, R.; Dilger, R.N. The role of oligosaccharides and polysaccharides of xylan and mannan in gut health of monogastric animals. J. Nutr. Sci. 2020, 9, 9. [Google Scholar] [CrossRef]
- Zheng, L.-X.; Chen, X.-Q.; Cheong, K.-L. Current trends in marine algae polysaccharides: The digestive tract, microbial catabolism, and prebiotic potential. Int. J. Biol. Macromol. 2020, 151, 344–354. [Google Scholar] [CrossRef]
- Ates, Ö.; Oner, E.T.; Arga, K.Y. Genome-scale reconstruction of metabolic network for a halophilic extremophile, Chromohalobacter salexigens DSM 3043. BMC Syst. Biol. 2011, 5, 12. [Google Scholar] [CrossRef] [Green Version]
- Finore, I.; Di Donato, P.; Mastascusa, V.; Nicolaus, B.; Poli, A. Fermentation technologies for the optimization of marine microbial exopolysaccharide production. Mar. Drugs 2014, 12, 3005–3024. [Google Scholar] [CrossRef] [Green Version]
- Ates, O.; Arga, K.Y.; Oner, E.T. The stimulatory effect of mannitol on levan biosynthesis: Lessons from metabolic systems analysis of Halomonas smyrnensis AAD6T. Biotechnol. Prog. 2013, 29, 1386–1397. [Google Scholar] [CrossRef]
- Merritt, J.H.; Ollis, A.A.; Fisher, A.C.; DeLisa, M.P. Glycans-by-design: Engineering bacteria for the biosynthesis of complex glycans and glycoconjugates. Biotechnol. Bioeng. 2013, 110, 1550–1564. [Google Scholar] [CrossRef]
- Wang, Z.; Dordick, J.S.; Linhardt, R.J. Escherichia coli K5 heparosan fermentation and improvement by genetic engineering. Bioeng. Bugs 2011, 2, 63–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delbarre-Ladrat, C.; Sinquin, C.; Lebellenger, L.; Zykwinska, A.; Colliec-Jouault, S. Exopolysaccharides produced by marine bacteria and their applications as glycosaminoglycan-like molecules. Front. Chem. 2014, 2, 85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hartmann, M.; Lindhorst, T.K. The bacterial lectin FimH, a target for drug discovery–carbohydrate inhibitors of type 1 fimbriae-mediated bacterial adhesion. Eur. J. Org. Chem. 2011, 2011, 3583–3609. [Google Scholar] [CrossRef]
- Becer, C.R. The glycopolymer code: Synthesis of glycopolymers and multivalent carbohydrate–lectin interactions. Macromol. Rapid Commun. 2012, 33, 742–752. [Google Scholar] [CrossRef]
- Galan, M.C.; Benito-Alifonso, D.; Watt, G.M. Carbohydrate chemistry in drug discovery. Org. Biomol. Chem. 2011, 9, 3598–3610. [Google Scholar] [CrossRef]
- Xu, Y.; Liu, X. The versatility of carbohydrates in antimicrobial applications. J. Chin. Chem. Soc. 2020, 67, 2204–2207. [Google Scholar] [CrossRef]
- Taylor, M.E.; Drickamer, K. Introduction to Glycobiology; Oxford University Press: Oxford, UK, 2011; ISBN 0199569118. [Google Scholar]
- Rehm, B.H.A. Bacterial polymers: Biosynthesis, modifications and applications. Nat. Rev. Microbiol. 2010, 8, 578–592. [Google Scholar] [CrossRef]
- Jönsson, M.; Allahgholi, L.; Sardari, R.R.R.; Hreggviðsson, G.O.; Nordberg Karlsson, E. Extraction and modification of macroalgal polysaccharides for current and next-generation applications. Molecules 2020, 25, 930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kosaraju, S.L. Colon targeted delivery systems: Review of polysaccharides for encapsulation and delivery. Crit. Rev. Food Sci. Nutr. 2005, 45, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef] [PubMed]
- Begum, S.; Yuhana, N.Y.; Saleh, N.M.; Kamarudin, N.N.; Sulong, A.B. Review of chitosan composite as a heavy metal adsorbent: Material preparation and properties. Carbohydr. Polym. 2021, 259, 117613. [Google Scholar] [CrossRef] [PubMed]
- Schmid, J.; Sperl, N.; Sieber, V. A comparison of genes involved in sphingan biosynthesis brought up to date. Appl. Microbiol. Biotechnol. 2014, 98, 7719–7733. [Google Scholar] [CrossRef]
- Lamport, D.T.A. Structure and function of plant glycoproteins. In Carbohydrates: Structure and Function; Elsevier: Amsterdam, The Netherlands, 1980; pp. 501–541. [Google Scholar]
- Barreras, M.; Salinas, S.R.; Abdian, P.L.; Kampel, M.A.; Ielpi, L. Structure and mechanism of GumK, a membrane-associated glucuronosyltransferase. J. Biol. Chem. 2008, 283, 25027–25035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamat, S. Molecular Basis and Genetic Regulation of EPS. In Microbial Exopolysaccharides as Novel and Significant Biomaterials; Springer: Berlin/Heidelberg, Germany, 2021; pp. 45–83. [Google Scholar]
- Whitfield, C. Glycan chain-length control. Nat. Chem. Biol. 2010, 6, 403–404. [Google Scholar] [CrossRef]
- Tatnell, P.J.; Russell, N.J.; Gacesa, P. GDP-mannose dehydrogenase is the key regulatory enzyme in alginate biosynthesis in Pseudomonas aeruginosa: Evidence from metabolite studies. Microbiology 1994, 140, 1745–1754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trincone, A. Potential biocatalysts originating from sea environments. J. Mol. Catal. B Enzym. 2010, 66, 241–256. [Google Scholar] [CrossRef]
- Jiang, B.; Wang, L.; Zhu, M.; Wu, S.; Wang, X.; Li, D.; Liu, C.; Feng, Z.; Tian, B. Separation, structural characteristics and biological activity of lactic acid bacteria exopolysaccharides separated by aqueous two-phase system. LWT 2021, 147, 111617. [Google Scholar] [CrossRef]
- Xu, Y.; Cui, Y.; Yue, F.; Liu, L.; Shan, Y.; Liu, B.; Zhou, Y.; Lü, X. Exopolysaccharides produced by lactic acid bacteria and Bifidobacteria: Structures, physiochemical functions and applications in the food industry. Food Hydrocoll. 2019, 94, 475–499. [Google Scholar] [CrossRef]
- Gavande, P.V.; Basak, A.; Sen, S.; Lepcha, K.; Murmu, N.; Rai, V.; Mazumdar, D.; Saha, S.P.; Das, V.; Ghosh, S. Functional characterization of thermotolerant microbial consortium for lignocellulolytic enzymes with central role of Firmicutes in rice straw depolymerization. Sci. Rep. 2021, 11, 3032. [Google Scholar] [CrossRef] [PubMed]
- Chio, C.; Sain, M.; Qin, W. Lignin utilization: A review of lignin depolymerization from various aspects. Renew. Sustain. Energy Rev. 2019, 107, 232–249. [Google Scholar] [CrossRef]
- Murrey, H.E.; Hsieh-Wilson, L.C. The chemical neurobiology of carbohydrates. Chem. Rev. 2008, 108, 1708–1731. [Google Scholar] [CrossRef] [Green Version]
- Farrokhi, N.; Burton, R.A.; Brownfield, L.; Hrmova, M.; Wilson, S.M.; Bacic, A.; Fincher, G.B. Plant cell wall biosynthesis: Genetic, biochemical and functional genomics approaches to the identification of key genes. Plant Biotechnol. J. 2006, 4, 145–167. [Google Scholar] [CrossRef]
- Thibodeaux, C.J.; Melançon III, C.E.; Liu, H. Natural-product sugar biosynthesis and enzymatic glycodiversification. Angew. Chemie Int. Ed. 2008, 47, 9814–9859. [Google Scholar] [CrossRef] [Green Version]
- Jamieson, C.S.; Misa, J.; Tang, Y.; Billingsley, J.M. Biosynthesis and synthetic biology of psychoactive natural products. Chem. Soc. Rev. 2021, 50, 6950–7008. [Google Scholar] [CrossRef]
- Winter, J.M.; Tang, Y. Synthetic biological approaches to natural product biosynthesis. Curr. Opin. Biotechnol. 2012, 23, 736–743. [Google Scholar] [CrossRef] [Green Version]
- Cameron, D.E.; Bashor, C.J.; Collins, J.J. A brief history of synthetic biology. Nat. Rev. Microbiol. 2014, 12, 381–390. [Google Scholar] [CrossRef]
- Church, G.M.; Elowitz, M.B.; Smolke, C.D.; Voigt, C.A.; Weiss, R. Realizing the potential of synthetic biology. Nat. Rev. Mol. Cell Biol. 2014, 15, 289–294. [Google Scholar] [CrossRef]
- Chien, L.; Lee, C. Enhanced Hyaluronic Acid Production in Bacillussubtilis by Coexpressing Bacterial Hemoglobin. Biotechnol. Prog. 2007, 23, 1017–1022. [Google Scholar]
- Cimini, D.; De Rosa, M.; Schiraldi, C. Production of glucuronic acid-based polysaccharides by microbial fermentation for biomedical applications. Biotechnol. J. 2012, 7, 237–250. [Google Scholar] [CrossRef]
- Leroux, M.; Michaud, J.; Bayma, E.; Armand, S.; Drouillard, S.; Priem, B. Misincorporation of Galactose by Chondroitin Synthase of Escherichia coli K4: From Traces to Synthesis of Chondbiuronan, a Novel Chondroitin-Like Polysaccharide. Biomolecules 2020, 10, 1667. [Google Scholar] [CrossRef]
- Roman, E.; Roberts, I.; Lidholt, K.; Kusche-Gullberg, M. Overexpression of UDP-glucose dehydrogenase in Escherichia coli results in decreased biosynthesis of K5 polysaccharide. Biochem. J. 2003, 374, 767–772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kovarik, M.L.; Gach, P.C.; Ornoff, D.M.; Wang, Y.; Balowski, J.; Farrag, L.; Allbritton, N.L. Micro total analysis systems for cell biology and biochemical assays. Anal. Chem. 2012, 84, 516–540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, S.; Ullah, M.W.; Siddique, R.; Nabi, G.; Manan, S.; Yousaf, M.; Hou, H. Role of recombinant DNA technology to improve life. Int. J. Genom. 2016, 2016, 2405954. [Google Scholar] [CrossRef]
- Park, T.G.; Jeong, J.H.; Kim, S.W. Current status of polymeric gene delivery systems. Adv. Drug Deliv. Rev. 2006, 58, 467–486. [Google Scholar] [CrossRef]
- Vu, B.; Chen, M.; Crawford, R.J.; Ivanova, E.P. Bacterial extracellular polysaccharides involved in biofilm formation. Molecules 2009, 14, 2535–2554. [Google Scholar] [CrossRef]
- Horton, R.M.; Cai, Z.; Ho, S.N.; Pease, L.R. Gene splicing by overlap extension: Tailor-made genes using the polymerase chain reaction. Biotechniques 2013, 54, 129–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schanté, C.E.; Zuber, G.; Herlin, C.; Vandamme, T.F. Chemical modifications of hyaluronic acid for the synthesis of derivatives for a broad range of biomedical applications. Carbohydr. Polym. 2011, 85, 469–489. [Google Scholar] [CrossRef]
- Lundqvist, L.C.E.; Jam, M.; Barbeyron, T.; Czjzek, M.; Sandström, C. Substrate specificity of the recombinant alginate lyase from the marine bacteria Pseudomonas alginovora. Carbohydr. Res. 2012, 352, 44–50. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Li, N.; Yang, S.; Yu, Y.; Han, Z.; Li, L.; Mou, H. Study on expression and action mode of recombinant alginate lyases based on conserved domains reconstruction. Appl. Microbiol. Biotechnol. 2019, 103, 807–817. [Google Scholar] [CrossRef]
- Rao, M.G.; Bharathi, P.; Akila, R.M. A comprehensive review on biopolymers. Sci. Revs. Chem. Commun 2014, 4, 61–68. [Google Scholar]
- Das, B.; Paul, S.; Sharma, H.K. A Review on Bio-Polymers Derived from Animal Sources with Special Reference to their Potential Applications. J. Drug Deliv. Ther. 2021, 11, 209–223. [Google Scholar]
- McCart, J.A.; Ward, J.M.; Lee, J.; Hu, Y.; Alexander, H.R.; Libutti, S.K.; Moss, B.; Bartlett, D.L. Systemic cancer therapy with a tumor-selective vaccinia virus mutant lacking thymidine kinase and vaccinia growth factor genes. Cancer Res. 2001, 61, 8751–8757. [Google Scholar]
- Brown, C.K.; Gu, Z.-Y.; Matsuka, Y.V.; Purushothaman, S.S.; Winter, L.A.; Cleary, P.P.; Olmsted, S.B.; Ohlendorf, D.H.; Earhart, C.A. Structure of the streptococcal cell wall C5a peptidase. Proc. Natl. Acad. Sci. USA 2005, 102, 18391–18396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, Z.; Shin, H.-D.; Chen, R. A recombinant E. coli bioprocess for hyaluronan synthesis. Appl. Microbiol. Biotechnol. 2009, 84, 63–69. [Google Scholar] [CrossRef]
- Fialho, A.M.; Moreira, L.M.; Granja, A.T.; Popescu, A.O.; Hoffmann, K.; Sá-Correia, I. Occurrence, production, and applications of gellan: Current state and perspectives. Appl. Microbiol. Biotechnol. 2008, 79, 889–900. [Google Scholar] [CrossRef]
- Kumar, A.S.; Mody, K. Microbial exopolysaccharides: Variety and potential applications. In Microbial Production of Biopolymers and Polymer Precursors: Applications and Perspectives; Caister Academic Press: Norfolk, UK, 2009; pp. 229–253. [Google Scholar]
- Yin, G.-Z.; Zhang, W.-B.; Cheng, S.Z.D. Giant molecules: Where chemistry, physics, and bio-science meet. Sci. China Chem. 2017, 60, 338–352. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Salas, C.; Rojas, O.J. Curdlan in fibers as carriers of tetracycline hydrochloride: Controlled release and antibacterial activity. Carbohydr. Polym. 2016, 154, 194–203. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Abdelgawad, A.M.; Tripathi, A.; Rojas, O.J. Curdlan cryogels reinforced with cellulose nanofibrils for controlled release. J. Environ. Chem. Eng. 2017, 5, 5754–5761. [Google Scholar] [CrossRef]
- El-Naggar, M.E. Synthesis, Drying Process and Medical Application of Polysaccharide-Based Aerogels. Int. J. Biol. Macromol. 2020, 145, 1115–1128. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, T.I.; El-Naggar, M.E.; Hussein, J.S.; El-Bana, M.; Emara, E.; El-Khayat, Z.; Fouda, M.M.; Ebaid, H.; Hebeish, A. Antidiabetic assessment; in vivo study of gold and core-shell silver-gold nanoparticles on streptozotocin-induced diabetic rats. Biomed. Pharmacother. 2016, 83, 865. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, A.L.; El-Naggar, M.E.; Shaheen, T.I.; Hassabo, A.G. Novel nano polymeric system containing biosynthesized core shell silver/silica nanoparticles for functionalization of cellulosic based material. Microsyst. Technol. 2016, 22, 979–992. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Latif, Y.A.; Medhat, D.; El Bana, M.; Refaat, E.; Morsy, S. Solvent-free and one-pot synthesis of silver and zinc oxide nanoparticles: Activity toward cell membrane component and insulin signaling pathway in experimental diabetes. Colloids Surf. B Biointerfaces 2018, 170, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Prausnitz, M.R.; Mitragotri, S.; Langer, R. Current status and future potential of transdermal drug delivery. Nat. Rev. Drug Discov. 2004, 3, 115–124. [Google Scholar] [CrossRef]
- Patra, J.K.; Das, G.; Fraceto, L.F.; Campos, E.V.R.; del Pilar Rodriguez-Torres, M.; Acosta-Torres, L.S.; Diaz-Torres, L.A.; Grillo, R.; Swamy, M.K.; Sharma, S. Nano based drug delivery systems: Recent developments and future prospects. J. Nanobiotechnol. 2018, 16, 71. [Google Scholar] [CrossRef] [Green Version]
- Selvasudha, N.; Koumaravelou, K. The multifunctional synergistic effect of chitosan on simvastatin loaded nanoparticulate drug delivery system. Carbohydr. Polym. 2017, 163, 70–80. [Google Scholar] [CrossRef]
- Maheshwari, R.; Sharma, P.; Seth, A.; Taneja, N.; Tekade, M.; Tekade, R.K. Drug Disposition Considerations in Pharmaceutical Product. In Dosage Form Design Considerations; Elsevier: Amsterdam, The Netherlands, 2018; pp. 337–369. [Google Scholar]
- Lee, H.; Sundaram, J.; Mani, S. Production of Cellulose Nanofibrils and Their Application to Food: A Review; Prasad, R., Kumar, V., Kumar, M., Eds.; Springer: Singapore, 2017; ISBN 9789811046780. [Google Scholar] [CrossRef]
- Ramalingam, C.; Priya, J.; Mundra, S. Applications of microbial polysaccharides in food industry. Int. J. Pharm. Sci. Rev. Res. 2014, 27, 322–324. [Google Scholar]
- Sworn, G. Xanthan gum. In Handbook of Hydrocolloids; Woodhead Publishing: Sawston, UK, 2021; pp. 833–853. [Google Scholar] [CrossRef]
- Preichardt, L.D.; Vendruscolo, C.T.; Gularte, M.A.; Moreira, A.D. The role of xanthan gum in the quality of gluten free cakes: Improved bakery products for coeliac patients. Int. J. Food Sci. Technol. 2011, 46, 2591–2597. [Google Scholar] [CrossRef]
- Moritaka, H.; Naito, S.; Nishinari, K.; Ishihara, M.; Fukuba, H. Effects of gellan gum, citric acid and sweetener on the texture of lemon jelly. J. Texture Stud. 1999, 30, 29–41. [Google Scholar] [CrossRef]
- Duran, E.; Costell, E.; Izquierdo, L.; Duran, L. Low sugar bakery jams with gellan gum—guar gum mixtures. Influence of composition on texture. Food Hydrocoll. 1994, 8, 373–381. [Google Scholar] [CrossRef]
- Bajaj, I.B.; Survase, S.A.; Saudagar, P.S.; Singhal, R.S. Gellan gum: Fermentative production, downstream processing and applications. Food Technol. Biotechnol. 2007, 45, 341–354. [Google Scholar]
- Totosaus, A.; Perez-Chabela, M.L. Textural properties and microstructure of low-fat and sodium-reduced meat batters formulated with gellan gum and dicationic salts. LWT-Food Sci. Technol. 2009, 42, 563–569. [Google Scholar] [CrossRef]
- Nieto, M.B. Structure and function of polysaccharide gum-based edible films and coatings. In Edible Films and Coatings for Food Applications; Springer: Berlin/Heidelberg, Germany, 2009; pp. 57–112. [Google Scholar]
- Tsujisaka, Y.; Mitsuhashi, M. Pullulan. In Industrial Gums; Elsevier: Amsterdam, The Netherlands, 1993; pp. 447–460. [Google Scholar]
- Kothari, D.; Das, D.; Patel, S.; Goyal, A. Dextran and food application. In Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2014; pp. 1–16. [Google Scholar]
- McCurdy, R.D.; Goff, H.D.; Stanley, D.W. Properties of dextran as a cryoprotectant in ice cream. Food Hydrocoll. 1994, 8, 625–633. [Google Scholar] [CrossRef]
- Shi, Z.; Zhang, Y.; Phillips, G.O.; Yang, G. Utilization of bacterial cellulose in food. Food Hydrocoll. 2014, 35, 539–545. [Google Scholar] [CrossRef]
- Sukumaran, S. Studies on Sporulation in Some Commercially Important Marine Algae of Mandapam Coast; Central Marine Fisheries Research Institute: Kochi, India, 2000. [Google Scholar]
- Helgerud, T.; Gaserod, O.; Fjæreide, T.; Andersen, P.; Larsen, C. 4. Alginates. In Food Stabilisers, Thickeners and Gelling Agents; Wiley-Blackwell: Oxford, UK, 2010; pp. 50–72. [Google Scholar]
- Thomas, F.; Lundqvist, L.C.E.; Jam, M.; Jeudy, A.; Barbeyron, T.; Sandström, C.; Michel, G.; Czjzek, M. Comparative characterization of two marine alginate lyases from Zobellia galactanivorans reveals distinct modes of action and exquisite adaptation to their natural substrate. J. Biol. Chem. 2013, 288, 23021–23037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuasa, M.; Tagawa, Y.; Tominaga, M. The texture and preference of “mentsuyu (Japanese noodle soup base) caviar” prepared from sodium alginate and calcium lactate. Int. J. Gastron. Food Sci. 2019, 18, 100178. [Google Scholar] [CrossRef]
- Ahari, H.; Fahimdanesh, M.; Khosravi Zanjani, M.A.; Anvar, A.; Shokri, E. Survival of alginate-prebiotic microencapsulated Lactobacillus acidophilus in mayonnaise sauce. Iran. J. Vet. Med. 2012, 6, 259–264. [Google Scholar]
- Petracci, M.; Bianchi, M.; Mudalal, S.; Cavani, C. Functional ingredients for poultry meat products. Trends food Sci. Technol. 2013, 33, 27–39. [Google Scholar] [CrossRef]
- Storebakken, T.; Austreng, E. Binders in fish feeds: II. Effect of different alginates on the digestibility of macronutrients in rainbow trout. Aquaculture 1987, 60, 121–131. [Google Scholar] [CrossRef]
- Marguet, M.; Sandre, O.; Lecommandoux, S. Polymersomes in “gelly” polymersomes: Toward structural cell mimicry. Langmuir 2012, 28, 2035–2043. [Google Scholar] [CrossRef]
- Hino, T.; Ishimoto, H.; Shimabayashi, S. Thermal gelation of aqueous curdlan suspension: Preparation of curdlan jelly. J. Pharm. Pharmacol. 2003, 55, 435–441. [Google Scholar] [CrossRef]
- Funami, T.; Yada, H.; Nakao, Y. Curdlan properties for application in fat mimetics for meat products. J. Food Sci. 1998, 63, 283–287. [Google Scholar] [CrossRef]
- Sadar, L.N. Rheological and Textural Characteristics of Copolymerized Hydrocolloidal Solutions Containing Curdlan Gum; University of Maryland: College Park, MD, USA, 2004; ISBN 0496261908. [Google Scholar]
- Nakao, Y.; Yamaguchi, T.; Taguchi, T. Preparations of freezable processed tofu and freeze-dried tofu by using curdlan. Nippon Shokuhin Kogyo Gakkaishi 1994, 41, 141–147. [Google Scholar] [CrossRef]
- Radwan, E.K.; El-Naggar, M.E.; Abdel-Karim, A.; Wassel, A.R. Multifunctional 3D cationic starch/nanofibrillated cellulose/silver nanoparticles nanocomposite cryogel: Synthesis, adsorption, and antibacterial characteristics. Int. J. Biol. Macromol. 2021, 189, 420–431. [Google Scholar] [CrossRef] [PubMed]
- El-Newehy, M.H.; El-Naggar, M.E.; Alotaiby, S.; El-Hamshary, H.; Moydeen, M.; Al-Deyab, S. Green Electrospining of Hydroxypropyl Cellulose Nanofibres for Drug Delivery Applications. J. Nanosci. Nanotechnol. 2018, 18, 805–814. [Google Scholar] [CrossRef]
- Yan, J.; Abdelgawad, A.M.; El-Naggar, M.E.; Rojas, O.J. Antibacterial activity of silver nanoparticles synthesized In-situ by solution spraying onto cellulose. Carbohydr. Polym. 2016, 147, 500–508. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Radwan, E.K.; El-Wakeel, S.T.; Kafafy, H.; Gad-Allah, T.A.; El-Kalliny, A.S.; Shaheen, T.I. Synthesis, characterization and adsorption properties of microcrystalline cellulose based nanogel for dyes and heavy metals removal. Int. J. Biol. Macromol. 2018, 113, 248–258. [Google Scholar] [CrossRef]
- Hussein, J.; El-Banna, M.; Razik, T.A.; El-Naggar, M.E. Biocompatible zinc oxide nanocrystals stabilized via hydroxyethyl cellulose for mitigation of diabetic complications. Int. J. Biol. Macromol. 2018, 107, 748–754. [Google Scholar] [CrossRef]
- Abdelrahman, M.S.; Khattab, T.A.; Aldalbahi, A.; Hatshan, M.R.; El-Naggar, M.E. Facile development of microporous cellulose acetate xerogel immobilized with hydrazone probe for real time vapochromic detection of toxic ammonia. J. Environ. Chem. Eng. 2020, 8, 104573. [Google Scholar] [CrossRef]
- Sharaf, S.; El-Naggar, M.E. Eco-friendly technology for preparation, characterization and promotion of honey bee propolis extract loaded cellulose acetate nanofibers in medical domains. Cellulose 2018, 25, 5195–5204. [Google Scholar] [CrossRef]
- Abdelgawad, A.M.; El-Naggar, M.E.; Elsherbiny, D.A.; Ali, S.; Abdel-Aziz, M.S.; Abd-Elmoneam, Y.K. Antibacterial carrageenan/cellulose-nanocrystal system loaded with silver nanoparticles, prepared via solid-state technique. J. Environ. Chem. Eng. 2020, 8, 104276. [Google Scholar] [CrossRef]
- El-Naggar, M.E.; Hasanin, M.; Youssef, A.M.; Aldalbahi, A.; El-Newehy, M.H.; Abdelhameed, R.M. Hydroxyethyl cellulose/bacterial cellulose cryogel dopped silver@titanium oxide nanoparticles: Antimicrobial activity and controlled release of Tebuconazole fungicide. Int. J. Biol. Macromol. 2020, 165, 1010–1021. [Google Scholar] [CrossRef]
- Sanz, T.; Laguna, L.; Salvador, A. Biscuit dough structural changes during heating: Influence of shortening and cellulose ether emulsions. LWT-Food Sci. Technol. 2015, 62, 962–969. [Google Scholar] [CrossRef] [Green Version]
- El-Naggar, M.E.; Abd-Al-Aleem, A.H.; Abu-Saied, M.A.; Youssef, A.M. Synthesis of environmentally benign antimicrobial dressing nanofibers based on polycaprolactone blended with gold nanoparticles and spearmint oil nanoemulsion. J. Mater. Res. Technol. 2021, 15, 3447–3460. [Google Scholar] [CrossRef]
- Sun, H.; Zhu, X.; Li, C.; Ma, Z.; Han, X.; Luo, Y.; Yang, L.; Yu, J.; Miao, Y. Xanthomonas effector XopR hijacks host actin cytoskeleton via complex coacervation. Nat. Commun. 2021, 12, 4064. [Google Scholar] [CrossRef]
- Mielnichuk, N.; Bianco, M.I.; Yaryura, P.M.; Bertani, R.P.; Toum, L.; Daglio, Y.; Colonnella, M.A.; Lizarraga, L.; Castagnaro, A.P.; Vojnov, A.A. Virulence factors analysis of native isolates of Xanthomonas albilineans and Xanthomonas sacchari from Tucumán, Argentina, reveals differences in pathogenic strategies. Plant Pathol. 2021, 70, 1072–1084. [Google Scholar] [CrossRef]
- Barak, S.; Mudgil, D.; Taneja, S. Exudate gums: Chemistry, properties and food applications–a review. J. Sci. Food Agric. 2020, 100, 2828–2835. [Google Scholar] [CrossRef]
- Yang, X.; Li, A.; Li, X.; Sun, L.; Guo, Y. An overview of classifications, properties of food polysaccharides and their links to applications in improving food textures. Trends Food Sci. Technol. 2020, 102, 1–15. [Google Scholar] [CrossRef]
- Zia, K.M.; Tabasum, S.; Khan, M.F.; Akram, N.; Akhter, N.; Noreen, A.; Zuber, M. Recent trends on gellan gum blends with natural and synthetic polymers: A review. Int. J. Biol. Macromol. 2018, 109, 1068–1087. [Google Scholar] [CrossRef]
- Medhat, D.; Hussein, J.; El-Naggar, M.E.; Attia, M.F.; Anwar, M.; Latif, Y.A.; Booles, H.F.; Morsy, S.; Farrag, A.R.; Khalil, W.K.B.; et al. Effect of Au-dextran NPs as anti-tumor agent against EAC and solid tumor in mice by biochemical evaluations and histopathological investigations. Biomed. Pharmacother. 2017, 91, 1006–1016. [Google Scholar] [CrossRef]
- Elnaggar, M.; Emam, H.; Fathalla, M.; Abdel-Aziz, M.; Zahran, M. Chemical synthesis of silver nanoparticles in its solid state: Highly efficient antimicrobial cotton fabrics for wound healing properties. Egypt. J. Chem. 2021, 64, 2697–2709. [Google Scholar] [CrossRef]
- Hussein, J.; El-Naggar, M.E.; Fouda, M.M.G.; Othman, S.I.; Allam, A.A.; Nadwa, E.H.; Rashwan, E.K.; Hendawy, O.M. Eco-friendly Microwave Synthesis of Gold Nanoparticles for Attenuation of Brain Dysfunction in Diabetic Rats. J. Clust. Sci. 2021, 132, 423–435. [Google Scholar] [CrossRef]
- Wang, Y.; Maina, N.H.; Coda, R.; Katina, K. Challenges and opportunities for wheat alternative grains in breadmaking: Ex-situ- versus in-situ-produced dextran. Trends Food Sci. Technol. 2021, 113, 232–244. [Google Scholar] [CrossRef]
- Liu, P.; Shi, B.; Yue, C.; Gao, G.; Li, P.; Yi, H.; Li, M.; Wang, B.; Ma, Y.; Cai, L. Dextran-based redox-responsive doxorubicin prodrug micelles for overcoming multidrug resistance. Polym. Chem. 2013, 4, 5793–5799. [Google Scholar] [CrossRef]
- Singh, R.S.; Kaur, N.; Kennedy, J.F. Pullulan production from agro-industrial waste and its applications in food industry: A review. Carbohydr. Polym. 2019, 217, 46–57. [Google Scholar] [CrossRef] [PubMed]
- Saxelin, M.; Pessi, T.; Salminen, S. Fecal recovery following oral administration of Lactobacillus strain GG (ATCC 53103) in gelatine capsules to healthy volunteers. Int. J. Food Microbiol. 1995, 25, 199–203. [Google Scholar] [CrossRef]
- Hammerschmidt, S.; Wolff, S.; Hocke, A.; Rosseau, S.; Müller, E.; Rohde, M. Illustration of pneumococcal polysaccharide capsule during adherence and invasion of epithelial cells. Infect. Immun. 2005, 73, 4653–4667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, E.-S.A.; El-Sawi, E.-S.S.; Abdel-Glel, M.Y.; Mohamed, E.-D.N. Importance and possible applications of lactobacillus plantarum, azotobacter vinelandii and its exopolysaccharides to fresh and marine water fish farms. Egypt. J. Exp. Biol. 2011, 7, 187–196. [Google Scholar]
- Singh, P.; Magalhães, S.; Alves, L.; Antunes, F.; Miguel, M.; Lindman, B.; Medronho, B. Cellulose-based edible films for probiotic entrapment. Food Hydrocoll. 2019, 88, 68–74. [Google Scholar] [CrossRef]
- Li, W.; Liu, L.; Tian, H.; Luo, X.; Liu, S. Encapsulation of Lactobacillus plantarum in cellulose based microgel with controlled release behavior and increased long-term storage stability. Carbohydr. Polym. 2019, 223, 115065. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xie, M.; Nie, S. Recent trends and applications of polysaccharides for microencapsulation of probiotics. Food Front. 2020, 1, 45–59. [Google Scholar] [CrossRef] [Green Version]
- Rokka, S.; Rantamäki, P. Protecting probiotic bacteria by microencapsulation: Challenges for industrial applications. Eur. Food Res. Technol. 2010, 231, 1–12. [Google Scholar] [CrossRef]
- Ding, W.K.; Shah, N.P. An improved method of microencapsulation of probiotic bacteria for their stability in acidic and bile conditions during storage. J. Food Sci. 2009, 74, M53–M61. [Google Scholar] [CrossRef]
- Călinoiu, L.-F.; Ştefănescu, B.E.; Pop, I.D.; Muntean, L.; Vodnar, D.C. Chitosan coating applications in probiotic microencapsulation. Coatings 2019, 9, 194. [Google Scholar] [CrossRef] [Green Version]
- Chen, D.; Jin, D.; Huang, S.; Wu, J.; Xu, M.; Liu, T.; Dong, W.; Liu, X.; Wang, S.; Zhong, W. Clostridium butyricum, a butyrate-producing probiotic, inhibits intestinal tumor development through modulating Wnt signaling and gut microbiota. Cancer Lett. 2020, 469, 456–467. [Google Scholar] [CrossRef]
- Muñoz-Garcia, J.; Mazza, M.; Alliot, C.; Sinquin, C.; Colliec-Jouault, S.; Heymann, D.; Huclier-Markai, S. Antiproliferative properties of scandium exopolysaccharide complexes on several cancer cell lines. Mar. Drugs 2021, 19, 174. [Google Scholar] [CrossRef]
- Manivasagan, P.; Kim, S.-K. Extracellular polysaccharides produced by marine bacteria. Adv. Food Nutr. Res. 2014, 72, 79–94. [Google Scholar] [PubMed]
- McCarthy, R.R.; Ullah, M.W.; Booth, P.; Pei, E.; Yang, G. The use of bacterial polysaccharides in bioprinting. Biotechnol. Adv. 2019, 37, 107448. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, R.R.; Ullah, M.W.; Pei, E.; Yang, G. Antimicrobial inks: The anti-infective applications of bioprinted bacterial polysaccharides. Trends Biotechnol. 2019, 37, 1155–1159. [Google Scholar] [CrossRef]
- Muthukumaran, J.; Jha, N. Bacterial Polysaccharides utilization in Bioprinting. J. Crit. Rev. 2020, 7, 776–780. [Google Scholar]
- McIntosh, M.; Stone, B.A.; Stanisich, V.A. Curdlan and other bacterial (1 → 3)-β-D-glucans. Appl. Microbiol. Biotechnol. 2005, 68, 163–173. [Google Scholar] [CrossRef]
- Freimund, S.; Sauter, M.; Käppeli, O.; Dutler, H. A new non-degrading isolation process for 1, 3-β-d-glucan of high purity from baker’s yeast Saccharomyces cerevisiae. Carbohydr. Polym. 2003, 54, 159–171. [Google Scholar] [CrossRef]
- Pillai, C.K.S.; Paul, W.; Sharma, C.P. Chitin and chitosan polymers: Chemistry, solubility and fiber formation. Prog. Polym. Sci. 2009, 34, 641–678. [Google Scholar] [CrossRef]
- Onishi, H.; Nagai, T.; Machida, Y. Applications of chitin, chitosan, and their derivatives to drug carriers for microparticulated or conjugated drug delivery systems. In Applications of Chitin and Chitosan; CRC Press: Boca Raton, FL, USA, 2020; pp. 205–231. ISBN 100307281X. [Google Scholar]
- Yu, J.; Wu, N.; Zheng, X.; Zheng, M. Preparation of water-soluble chitosan/poly-gama-glutamic acid—tanshinone IIA encapsulation composite and its in vitro/in vivo drug release properties. Biomed. Phys. Eng. Express 2020, 6, 45020. [Google Scholar] [CrossRef]
- Sałek, K.; Euston, S.R. Sustainable microbial biosurfactants and bioemulsifiers for commercial exploitation. Process Biochem. 2019, 85, 143–155. [Google Scholar] [CrossRef]
- Das, S. Structural and mechanical characterization of biofilm-associated bacterial polymer in the emulsification of petroleum hydrocarbon. 3 Biotech 2021, 11, 239. [Google Scholar]
- Ask, M.; Ask, D.; Christiansson, R. Detection of borehole breakouts at the Forsmark site, Sweden. In Proceedings of the International Symposium on In-Situ Rock Stress, Trondheim, Norway, 19–21 June 2006; Lu, M., Li, C.C., Kjorholt, H., Dahle, H., Eds.; Routledge: Abingdon-on-Thames, UK, 2006; pp. 79–86. [Google Scholar]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Jiao, Q.; Wang, L.; Zhang, Y.; Jiang, B.; Li, D.; Feng, Z.; Liu, C. Preparation and evaluation of a novel high internal phase Pickering emulsion based on whey protein isolate nanofibrils derived by hydrothermal method. Food Hydrocoll. 2022, 123, 107180. [Google Scholar] [CrossRef]
- Jiao, Q.; Liu, Z.; Li, B.; Tian, B.; Zhang, N.; Liu, C.; Feng, Z.; Jiang, B. Development of Antioxidant and Stable Conjugated Linoleic Acid Pickering Emulsion with Protein Nanofibers by Microwave-Assisted Self-Assembly. Foods 2021, 10, 1892. [Google Scholar] [CrossRef] [PubMed]
- Singha, T.K. Microbial extracellular polymeric substances: Production, isolation and applications. IOSR J. Pharm. 2012, 2, 271–281. [Google Scholar]
- Anis, A.; Pal, K.; Al-Zahrani, S.M. Essential oil-containing polysaccharide-based edible films and coatings for food security applications. Polymers 2021, 13, 575. [Google Scholar] [CrossRef]
- Łupina, K.; Kowalczyk, D.; Zięba, E.; Kazimierczak, W.; Mężyńska, M.; Basiura-Cembala, M.; Wiącek, A.E. Edible films made from blends of gelatin and polysaccharide-based emulsifiers—A comparative study. Food Hydrocoll. 2019, 96, 555–567. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, W.; Tian, B.; Li, D.; Liu, C.; Jiang, B.; Feng, Z. Preparation and characterization of coating based on protein nanofibers and polyphenol and application for salted duck egg yolks. Foods 2020, 9, 449. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Shi, L.; Ren, Z.; Hao, G.; Chen, J.; Weng, W. Characterization of emulsion films prepared from soy protein isolate at different preheating temperatures. J. Food Eng. 2021, 309, 110697. [Google Scholar] [CrossRef]
- Rawlings, D.E.; Johnson, D.B. The microbiology of biomining: Development and optimization of mineral-oxidizing microbial consortia. Microbiology 2007, 153, 315–324. [Google Scholar] [CrossRef] [Green Version]
- Shukla, S.K.; Khan, A.; Rao, T.S. Biofilm extracellular polymeric substances-based bioremediation of radionuclides. In Microbial and Natural Macromolecules; Elsevier: Amsterdam, The Netherlands, 2021; pp. 751–773. [Google Scholar]
- Fang, K.; Park, O.-J.; Hong, S.H. Controlling biofilms using synthetic biology approaches. Biotechnol. Adv. 2020, 40, 107518. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Paul, D.; Jain, R.K. Biofilms: Implications in bioremediation. Trends Microbiol. 2006, 14, 389–397. [Google Scholar] [CrossRef] [PubMed]
- Pal, A.; Paul, A.K. Microbial extracellular polymeric substances: Central elements in heavy metal bioremediation. Indian J. Microbiol. 2008, 48, 49. [Google Scholar] [CrossRef] [Green Version]
- Dabrowska, M.; Debiec-Andrzejewska, K.; Andrunik, M.; Bajda, T.; Drewniak, L. The biotransformation of arsenic by spent mushroom compost–An effective bioremediation agent. Ecotoxicol. Environ. Saf. 2021, 213, 112054. [Google Scholar] [CrossRef] [PubMed]
- Kushkevych, I.; Dordević, D.; Vítězová, M.; Rittmann, S.K.-M. Environmental Impact of Sulfate-Reducing Bacteria, Their Role in Intestinal Bowel Diseases, and Possible Control by Bacteriophages. Appl. Sci. 2021, 11, 735. [Google Scholar] [CrossRef]
- Sharma, P. Efficiency of bacteria and bacterial assisted phytoremediation of heavy metals: An update. Bioresour. Technol. 2021, 328, 124835. [Google Scholar] [CrossRef] [PubMed]
- Sharma, P.; Pandey, A.K.; Kim, S.-H.; Singh, S.P.; Chaturvedi, P.; Varjani, S. Critical review on microbial community during in-situ bioremediation of heavy metals from industrial wastewater. Environ. Technol. Innov. 2021, 24, 101826. [Google Scholar] [CrossRef]




| Genetic Engineering Applications | Microorganisms Involved | References |
|---|---|---|
| Recombinant Hyaluronan/hyaluronic acid (HA) production | Bacillus subtilis E coli JM109 | [97] [98] |
| Recombinant Alginate and alginate production | P. aeruginosa | [69] |
| Intracellular versus extracellular production of biopolymers | - | [95,96] |
| Polysaccharide | Types of Utilized Foods | Required Concentration (%) | Functionality | References |
|---|---|---|---|---|
| Cellulose | dough-based products | texture modifier | [112] | |
| creamed soups | 0.75 | Thickening agent | [112] | |
| Xanthan gum | dressings for salads | 0.1–0.5 | Suspending agent, dispersant, and emulsion stabilizer | [113,114,115] |
| Mixes that are dry | 0.05–0.2 | Disperses in hot or cold water with ease. | ||
| Sauces, toppings, relishes, and syrups | 0.05–0.2 | Heat retention and uniform viscosity are both qualities of a thickener. | ||
| Beverages | 0.05–0.2 | Stabilizing agent | ||
| Milk products | 0.2–0.5 | Stabilizing agent; controlling the viscosity control of mixture | ||
| Baked foods | 0.1–0.4 | Stabilizing agent | ||
| Frozen foods | 0.05–0.2 | Improves the stability of the freeze-thaw cycle | ||
| Gellan | Jellies Jams Confectionery Processed foods Processed meats Icings Pie fillings | 0.15–0.2 0.12–0.3 0.8–1 0.2–0.3 0.1–1 0.05–0.12 0.25–0.35 | Agent for gelling Spreads with few calories Fruit and vegetable gelling Modification of texture Agent for coating Texturizer | [99,116,117,118,119] |
| Pullulan | Edible Films in confectionary decoration Snack Foods | 5–10 | Low oxygen permeability edible films, bioadhesive stability at high pH, and low viscosity NaCl | [120,121] |
| Dextran | Bakery products | 2 | Distinctive dough-mixing characteristics | [122,123] |
| Ice cream, Frozen and dried foods | 2–4 | Beneficial viscosity properties Film of dextran used in frozen foods | ||
| Xylinan/Acetobacterxylinum cellulose | Confectionery product-Nata de coco | Agent for gelling and controlling the viscosity | [113,124] | |
| Alginates | Confectionery, Dairy products Beverages, Jams, Soups, Sauces, Meat, Fish, and gellies | 0.3 | Gelling agent, thickener, and stabilizer | [125,126,127,128,129,130,131,132] |
| Curdlan | Gellies | 1–5 | Agent for gelling | [102,103,133,134,135,136,137] |
| Processed meats | 1–10 | Agent for gelling | ||
| Processed meats | 0.1–1 | Modification of texture | ||
| Sauces | 0.2–0.7 | Improving the mixture viscosity | ||
| Freeze-dried foods | 0.5–1 | Improving the mixture rehydration |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahmoud, Y.A.-G.; El-Naggar, M.E.; Abdel-Megeed, A.; El-Newehy, M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers 2021, 13, 4136. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234136
Mahmoud YA-G, El-Naggar ME, Abdel-Megeed A, El-Newehy M. Recent Advancements in Microbial Polysaccharides: Synthesis and Applications. Polymers. 2021; 13(23):4136. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234136
Chicago/Turabian StyleMahmoud, Yehia A.-G., Mehrez E. El-Naggar, Ahmed Abdel-Megeed, and Mohamed El-Newehy. 2021. "Recent Advancements in Microbial Polysaccharides: Synthesis and Applications" Polymers 13, no. 23: 4136. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13234136