Ultraviolet-C as a Viable Reprocessing Method for Disposable Masks and Filtering Facepiece Respirators
Abstract
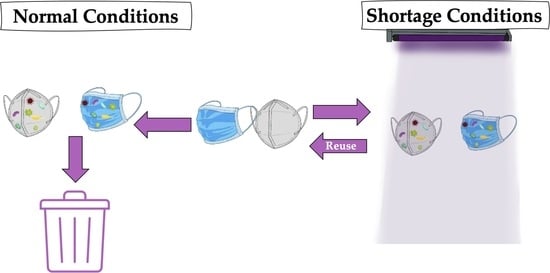
:1. Introduction
2. UV-C Sterilization in Masks and Filtering Facepiece Respirators
3. UV-C’s Germicidal Capability
4. UV-C’s Additional Advantages
5. UV-C’s Disadvantages
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Mills, D.; Harnish, D.A.; Lawrence, C.; Sandoval-Powers, M.; Heimbuch, B.K. Ultraviolet germicidal irradiation of influenza-contaminated N95 filtering facepiece respirators. Am. J. Infect. Control 2018, 46, e49–e55. [Google Scholar] [CrossRef] [Green Version]
- Rowan, N.J.; Laffey, J.G. Challenges and solutions for addressing critical shortage of supply chain for personal and protective equipment (PPE) arising from Coronavirus disease (COVID19) pandemic—Case study from the Republic of Ireland. Sci. Total Environ. 2020, 725, 138532. [Google Scholar] [CrossRef]
- Derraik, J.G.B.; Anderson, W.A.; Connelly, E.A.; Anderson, Y.C. Rapid Review of SARS-CoV-1 and SARS-CoV-2 Viability, Susceptibility to Treatment, and the Disinfection and Reuse of PPE, Particularly Filtering Facepiece Respirators. Int. J. Environ. Res. Public Health 2020, 17, 6117. [Google Scholar] [CrossRef]
- Livingston, E.H.; Desai, A.; Berkwits, M. Sourcing Personal Protective Equipment During the COVID-19 Pandemic. JAMA 2020, 323, 1912–1914. [Google Scholar] [CrossRef] [Green Version]
- Deskins, A. Attention Nurses, Doctors, First Responders, Healthcare Providers and Anyone Re-Using an N95 Mask or Making One. 2020. Available online: https://i0.wp.com/www.sages.org/wp-content/uploads/2020/03/reuse-n95.png?ssl=1 (accessed on 5 February 2021).
- Fisher, E.; Shaffer, R. A method to determine the available UV-C dose for the decontamination of filtering facepiece respirators. J. Appl. Microbiol. 2010, 110, 287–295. [Google Scholar] [CrossRef]
- Lindsley, W.G.; Martin, S.B., Jr.; Thewlis, R.E.; Sarkisian, K.; Nwoko, J.O.; Mead, K.R.; Noti, J.D. Effects of Ultraviolet Germicidal Irradiation (UVGI) on N95 Respirator Filtration Performance and Structural Integrity. J. Occup. Environ. Hyg. 2015, 12, 509–517. [Google Scholar] [CrossRef] [PubMed]
- Rubio-Romero, J.C.; Pardo-Ferreira, M.D.C.; Torrecilla-García, J.A.; Calero-Castro, S. Disposable masks: Disinfection and sterilization for reuse, and non-certified manufacturing, in the face of shortages during the COVID-19 pandemic. Saf. Sci. 2020, 129, 104830. [Google Scholar] [CrossRef] [PubMed]
- Czubryt, M.; Stecy, T.; Popke, E.; Aitken, R.; Jabusch, K.; Pound, R.; Lawes, P.; Ramjiawan, B.; Pierce, G. N95 mask reuse in a major urban hospital: COVID-19 response process and procedure. J. Hosp. Infect. 2020, 106, 277–282. [Google Scholar] [CrossRef] [PubMed]
- Harskamp, R.E.; Van Straten, B.; Bouman, J.; Santvoort, B.V.M.-V.; Dobbelsteen, J.J.V.D.; Van Der Sijp, J.R.; Horeman, T. Reprocessing filtering facepiece respirators in primary care using medical autoclave: Prospective, bench-to-bedside, single-centre study. BMJ Open 2020, 10, e039454. [Google Scholar] [CrossRef] [PubMed]
- Ranney, M.L.; Griffeth, V.; Jha, A.K. Critical Supply Shortages—The Need for Ventilators and Personal Protective Equipment during the Covid-19 Pandemic. N. Engl. J. Med. 2020, 382, e41. [Google Scholar] [CrossRef] [PubMed]
- Paul, D.; Gupta, A.; Maurya, A.K. Exploring options for reprocessing of N95 Filtering Facepiece Respirators (N95-FFRs) amidst COVID-19 pandemic: A systematic review. PLoS ONE 2020, 15, e0242474. [Google Scholar] [CrossRef]
- Pascoe, M.; Robertson, A.; Crayford, A.; Durand, E.; Steer, J.; Castelli, A.; Wesgate, R.; Evans, S.; Porch, A.; Maillard, J.-Y. Dry heat and microwave-generated steam protocols for the rapid decontamination of respiratory personal protective equipment in response to COVID-19-related shortages. J. Hosp. Infect. 2020, 106, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Daeschler, S.C.; Manson, N.; Joachim, K.; Chin, A.W.; Chan, K.; Chen, P.Z.; Jones, A.; Tajdaran, K.; Mirmoeini, K.; Zhang, J.J.; et al. Effect of moist heat reprocessing of N95 respirators on SARS-CoV-2 inactivation and respirator function. Can. Med. Assoc. J. 2020, 192, E1189–E1197. [Google Scholar] [CrossRef]
- Hinton, D.M. Emergency Use Authorization (EUA) for the Emergency Use of the Battelle CCDS Critical Care Decontamination System; FDA: Irvine, CL, USA, 2020.
- Hinton, D.M. Emergency Use Authorization (EUA) of the ASP STERRAD 100S, NX, and 100NX Sterilization Systems in the STERRAD 100S, NX Standard and 100NX Express Cycles, Respectively, for Decontamination of Compatible N95 Respirators; FDA: Irvine, CL, USA, 2020.
- Hinton, D.M. Emergency Use Authorization (EUA) for the Emergency Use of the STERIS V-PRO 1 Plus, maX, and maX2 Low Temperature Sterilization Systems; FDA: Irvine, CL, USA, 2020.
- Hinton, D.M. Emergency Use Authorization (EUA) for the Emergency Use of Stryker Instrument’s Sterizone VP4 Sterilizer; FDA: Irvine, CL, USA, 2020.
- World Health Organization (WHO). Rational Use of Personal Protective Equipment for Coronavirus Disease 2019 (COVID-19): Interim Guidance; WHO: Geneva, Switzerland, 2020; pp. 1–7. [Google Scholar]
- European Centre for Disease Prevention and Control. Cloth Masks and Mask Sterilisation as Options in Case of Shortage of Surgical Masks and Respirators; Technical Report; ECDC: Stockholm, Sweden, 2020. [Google Scholar]
- Centers for Disease Control and Contamination. Strategies for Optimizing the Supply of Facemasks. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/face-masks.html (accessed on 30 November 2020).
- Centers for Disease Control and Contamination. Implementing Filtering Facepiece Respirator (FFR) Reuse, Including Reuse after Decontamination, When There Are Known Shortages of N95 Respirators. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/decontamination-reuse-respirators.html (accessed on 30 November 2020).
- Centers for Disease Control and Contamination. Recommended Guidance for Extended Use and Limited Reuse of N95 Filtering Facepiece Respirators in Healthcare Settings. 2020. Available online: https://www.cdc.gov/niosh/topics/hcwcontrols/recommendedguidanceextuse.html (accessed on 30 November 2020).
- Centers for Disease Control and Contamination. Strategies for Optimizing the Supply of Isolation Gowns. 2020. Available online: https://www.cdc.gov/coronavirus/2019-ncov/hcp/ppe-strategy/isolation-gowns.html (accessed on 30 November 2020).
- McEvoy, B.; Rowan, N. Terminal sterilization of medical devices using vaporized hydrogen peroxide: A review of current methods and emerging opportunities. J. Appl. Microbiol. 2019, 127, 1403–1420. [Google Scholar] [CrossRef] [Green Version]
- Kenney, P.A.; Chan, B.J.; Kortright, K.; Cintron, M.; Havill, N.; Russi, M.; Epright, J.; Lee, L.; Balcezak, T.J.; Martinello, R.A. Hydrogen Peroxide Vapor sterilization of N95 respirators for reuse. medRxiv 2020. [Google Scholar] [CrossRef]
- Feldmann, F.; Shupert, W.L.; Haddock, E.; Twardoski, B.; Feldmann, H. Gamma Irradiation as an Effective Method for Inactivation of Emerging Viral Pathogens. Am. J. Trop. Med. Hyg. 2019, 100, 1275–1277. [Google Scholar] [CrossRef] [PubMed]
- Pierini, F.; Guglielmelli, A.; Urbanek, O.; Nakielski, P.; Pezzi, L.; Buda, R.; Lanzi, M.; Kowalewski, T.A.; De Sio, L. Thermoplasmonic-Activated Hydrogel Based Dynamic Light Attenuator. Adv. Opt. Mater. 2020, 8, 1–7. [Google Scholar] [CrossRef]
- Nakielski, P.; Pawłowska, S.; Rinoldi, C.; Ziai, Y.; De Sio, L.; Urbanek, O.; Zembrzycki, K.; Pruchniewski, M.; Lanzi, M.; Salatelli, E.; et al. Multifunctional Platform Based on Electrospun Nanofibers and Plasmonic Hydrogel: A Smart Nanostructured Pillow for Near-Infrared Light-Driven Biomedical Applications. ACS Appl. Mater. Interfaces 2020, 12, 54328–54342. [Google Scholar] [CrossRef]
- De Sio, L.; Ding, B.; Focsan, M.; Kogermann, K.; Pascoal-Faria, P.; Petronella, F.; Mitchell, G.; Zussman, E.; Pierini, F. Personalized Reusable Face Masks with Smart Nano-Assisted Destruction of Pathogens for COVID-19: A Visionary Road. Chem. Eur. J. 2020, 1–20. [Google Scholar] [CrossRef]
- Toomey, E.C.; Conway, Y.; Burton, C.; Smith, S.; Smalle, M.; Chan, X.-H.S.; Adisesh, A.; Tanveer, S.; Ross, L.; Thomson, I.; et al. Extended use or reuse of single-use surgical masks and filtering face-piece respirators during the coronavirus disease 2019 (COVID-19) pandemic: A rapid systematic review. Infect. Control Hosp. Epidemiol. 2021, 42, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Nogueira, M.S. Ultraviolet-based biophotonic technologies for control and prevention of COVID-19, SARS and related disorders. Photodiagn. Photodyn. Ther. 2020, 31, 101890. [Google Scholar] [CrossRef] [PubMed]
- Liao, L.; Xiao, W.; Zhao, M.; Yu, X.; Wang, H.; Wang, Q.; Chu, S.; Cui, Y. Can N95 Respirators Be Reused after Disinfection? How Many Times? ACS Nano 2020, 14, 6348–6356. [Google Scholar] [CrossRef] [PubMed]
- Ludwig-Begall, L.F.; Wielick, C.; Dams, L.; Nauwynck, H.; Demeuldre, P.-F.; Napp, A.; Laperre, J.; Haubruge, E.; Thiry, E. The use of germicidal ultraviolet light, vaporized hydrogen peroxide and dry heat to decontaminate face masks and filtering respirators contaminated with a SARS-CoV-2 surrogate virus. J. Hosp. Infect. 2020, 106, 577–584. [Google Scholar] [CrossRef] [PubMed]
- Cheok Hong, M.; Sook Lan, T.; Soo Zeng Fiona, P.; Wei Qi, L.; Shyue Wei, P.; Vidhya, N.; Geraldine, C.; Yu Hui Shermin, C. Surgical masks decontamination for reuse by members of the public: Feasibility study and development of home-based methods. Res. Sq. 2020, 1–15. [Google Scholar] [CrossRef]
- Ludwig-Begall, L.F.; Wielick, C.; Jolois, O.; Dams, L.; Razafimahefa, H.N.; Demeuldre, P.F.; Napp, A.; Laperre, J.; Farnir, F.; Thiry, E.; et al. From “don, doff, and discard” to “don, doff, and decontaminate”—Determination of filtering facepiece respirator and surgical mask integrity and inactivation of a SARS-CoV-2 surrogate and a small non-enveloped virus following multiple-cycles of vaporised hydrogen peroxide, ultraviolet germicidal irradiation and dry heat decontamination. medRxiv 2020, 1–13. [Google Scholar] [CrossRef]
- Cadnum, J.L.; Li, D.F.; Redmond, S.N.; John, A.R.; Pearlmutter, B.; Donskey, C.J. Effectiveness of Ultraviolet-C Light and a High-Level Disinfection Cabinet for Decontamination of N95 Respirators. Pathog. Immun. 2020, 5, 52–67. [Google Scholar] [CrossRef]
- Fischer, R.J.; Morris, D.H.; Van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Effectiveness of N95 Respirator Decontamination and Reuse against SARS-CoV-2 Virus. Emerg. Infect. Dis. 2020, 26, 2253–2255. [Google Scholar] [CrossRef]
- Hanyanunt, P.; Juntanawiwat, P.; Chatreewonanakul, T.; Potisuwan, P.; Simsiriporn, W.; Phondee, S.; Sungsirin, N.; Kesakomol, P.; Watanaveeradej, V.; Boonsiri, T. Effects of ultraviolet C (uvc) light and dry heat on filtration performance of N95 respirator mask. J. Southeast Asian Med. Res. 2020, 4, 48–52. [Google Scholar]
- Heimbuch, B.K.; Wallace, W.H.; Kinney, K.; Lumley, A.E.; Wu, C.-Y.; Woo, M.-H.; Wander, J.D. A pandemic influenza preparedness study: Use of energetic methods to decontaminate filtering facepiece respirators contaminated with H1N1 aerosols and droplets. Am. J. Infect. Control 2011, 39, e1–e9. [Google Scholar] [CrossRef]
- Kayani, B.J.; Weaver, D.T.; Gopalakrishnan, V.; King, E.S.; Dolson, E.; Krishnan, N.; Pelesko, J.; Scott, M.J.; Hitomi, M.; Cadnum, J.L.; et al. UV-C tower for point-of-care decontamination of filtering facepiece respirators. Am. J. Infect. Control 2020, 1–6. [Google Scholar] [CrossRef]
- Kumar, A.; Kasloff, S.B.; Leung, A.; Cutts, T.; Strong, J.E.; Hills, K.; Gu, F.X.; Chen, P.; Vazquez-Grande, G.; Rush, B.; et al. Decontamination of N95 masks for re-use employing 7 widely available sterilization methods. PLoS ONE 2020, 15, e0243965. [Google Scholar] [CrossRef]
- Lore, M.B.; Heimbuch, B.K.; Brown, T.L.; Wander, J.D.; Hinrichs, S.H. Effectiveness of Three Decontamination Treatments against Influenza Virus Applied to Filtering Facepiece Respirators. Ann. Occup. Hyg. 2011, 56, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muñoz, M.; Comtois-Bona, M.; Cortes, D.; Cimenci, C.E.; Du, Q.; Thompson, C.; Figueroa, J.D.; Franklin, V.; Liu, P.; Alarcon, E.I. Integrated photothermal decontamination device for N95 respirators. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Simmons, S.E.; Carrion, R.; Alfson, K.J.; Staples, H.M.; Jinadatha, C.; Jarvis, W.R.; Sampathkumar, P.; Chemaly, R.F.; Khawaja, F.; Povroznik, M.; et al. Deactivation of SARS-CoV-2 with pulsed-xenon ultraviolet light: Implications for environmental COVID-19 control. Infect. Control Hosp. Epidemiol. 2021, 42, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Singh, G.; Jorgenson, J.; Pringle, T.; Nelson, T.; Ramamoorthy, S. Monitoring SARS-CoV-2 decontamination by dry heat and ultraviolet treatment with a swine coronavirus as a surrogate. Infect. Prev. Pract. 2021, 3, 100103. [Google Scholar] [CrossRef]
- Smith, J.S.; Hanseler, H.; Welle, J.; Rattray, R.; Campbell, M.; Brotherton, T.; Moudgil, T.; Pack, T.F.; Wegmann, K.; Jensen, S.; et al. Effect of various decontamination procedures on disposable N95 mask integrity and SARS-CoV-2 infectivity. J. Clin. Transl. Sci. 2020, 1–5. [Google Scholar] [CrossRef]
- Vernez, D.; Save, J.; Oppliger, A.; Concha-Lozano, N.; Hopf, N.B.; Niculita-Hirzel, H.; Resch, G.; Michaud, V.; Dorange-Pattoret, L.; Charrière, N.; et al. Reusability of filtering facepiece respirators after decontamination through drying and germicidal UV irradiation. BMJ Glob. Health 2020, 5, e003110. [Google Scholar] [CrossRef]
- Vo, E.; Rengasamy, S.; Xu, S.; Horvatin, M.; Zhuang, Z. New technique to evaluate decontamination methods for filtering facepiece respirators. Am. J. Infect. Control 2021. [Google Scholar] [CrossRef]
- Vo, E.; Rengasamy, S.; Shaffer, R. Development of a Test System To Evaluate Procedures for Decontamination of Respirators Containing Viral Droplets. Appl. Environ. Microbiol. 2009, 75, 7303–7309. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigginton, K.R.; Arts, P.J.; Clack, H.L.; Fitzsimmons, W.J.; Gamba, M.; Harrison, K.R.; LeBar, W.; Lauring, A.S.; Li, L.; Roberts, W.W.; et al. Validation of N95 Filtering Facepiece Respirator Decontamination Methods Available at a Large University Hospital. Open Forum Infect. Dis. 2021, 8, ofaa610. [Google Scholar] [CrossRef]
- Woo, M.-H.; Grippin, A.; Anwar, D.; Smith, T.; Wu, C.-Y.; Wander, J.D. Effects of Relative Humidity and Spraying Medium on UV Decontamination of Filters Loaded with Viral Aerosols. Appl. Environ. Microbiol. 2012, 78, 5781–5787. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chotiprasitsakul, D.; Kitiyakara, T.; Jongkaewwattana, A.; Santanirand, P.; Jiaranaikulwanich, A.; Prahsam, C.; Wadwongsri, P.; Jeendum, N.; Watcharananan, S.P. Approach of using a household device in decontaminating respirators with ultraviolet C during the scarcity in the COVID-19 pandemic. Res. Sq. 2020, 1–12. [Google Scholar] [CrossRef]
- Fischer, R.J.; Morris, D.H.; van Doremalen, N.; Sarchette, S.; Matson, M.J.; Bushmaker, T.; Yinda, C.K.; Seifert, S.N.; Gamble, A.; Williamson, B.N.; et al. Assessment of N95 respirator decontamination and re-use for SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef] [Green Version]
- Golovkine, G.R.; Roberts, A.W.; Cooper, C.; Riano, S.; DiCiccio, A.M.; Worthington, D.L.; Clarkson, J.P.; Krames, M.; Zhang, J.; Gao, Y.; et al. Practical considerations for Ultraviolet-C radiation mediated decontamination of N95 respirator against SARS-CoV-2 virus. medRxiv 2020. [Google Scholar] [CrossRef]
- Ozog, D.M.; Sexton, J.Z.; Narla, S.; Pretto-Kernahan, C.; Mirabelli, C.; Lim, H.W.; Hamzavi, I.H.; Tibbetts, R.J.; Mi, Q.-S. The Effect of Ultraviolet C Radiation Against SARS-CoV-2 Inoculated N95. medRxiv 2020, 1–13. [Google Scholar] [CrossRef]
- Rathnasinghe, R.; Karlicek, R.F.; Schotsaert, M.; Koffas, M.; Arduini, B.; Jangra, S.; Wang, B.; Davis, J.L.; Alnaggar, M.; Costa, A.; et al. Scalable, effective, and rapid decontamination of SARS-CoV-2 con-taminated N95 respirators using germicidal Ultra-Violet C (UVC) irradiation device. medRxiv 2020. [Google Scholar] [CrossRef]
- Weaver, D.T.; McElvany, B.D.; Gopalakrishnan, V.; Card, K.J.; Crozier, D.; Dhawan, A.; Dinh, M.N.; Dolson, E.; Farrokhian, N.; Hitomi, M.; et al. UV decontamination of personal protective equipment with idle laboratory biosafety cabinets during the COVID-19 pandemic. medRxiv 2020, 1–18. [Google Scholar] [CrossRef] [Green Version]
- He, W.; Guo, Y.; Gao, H.; Liu, J.; Yue, Y.; Wang, J. Evaluation of Regeneration Processes for Filtering Facepiece Respirators in Terms of the Bacteria Inactivation Efficiency and Influences on Filtration Performance. ACS Nano 2020, 14, 13161–13171. [Google Scholar] [CrossRef] [PubMed]
- Lede, I.; Nolte, K.; Kroes, R.; Scalable, A. Method for Ultraviolet C Disinfection of Surgical Facemasks Type IIR and Filtering Facepiece Particle Respirators 1 and 2. Preprints 2020, 1–13. [Google Scholar] [CrossRef]
- Suen, C.Y.; Leung, H.H.; Lam, K.W.; Hung, K.P.S.; Chan, M.Y.; Kwan, J.K.C. Feasibility of reusing surgical mask under different disinfection treatments. medRxiv 2020. [Google Scholar] [CrossRef]
- Jung, S.; Hemmatian, T.; Song, E.; Lee, K.; Seo, D.; Yi, J.; Kim, J. Disinfection Treatments of Disposable Respirators Influencing the Bactericidal/Bacteria Removal Efficiency, Filtration Performance, and Structural Integrity. Polymers 2020, 13, 45. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.-H.; Tang, F.; Hung, P.-C.; Hua, Z.-C.; Lai, C.-Y. Relative survival of Bacillus subtilis spores loaded on filtering facepiece respirators after five decontamination methods. Indoor Air 2018, 28, 754–762. [Google Scholar] [CrossRef] [PubMed]
- Purschke, M.; Elsamaloty, M.; Wilde, J.; Starr, N.; Anderson, R.R.; Farinelli, W.; Sakamoto, F.; Tung, M.; Tam, J.; Hesselink, L.; et al. Construction and validation of UV-Cdecontamination cabinets for filteringfacepiece respirators. Appl. Opt. 2020, 59, 7585. [Google Scholar] [CrossRef]
- Kierat, W.; Augustyn, W.; Koper, P.; Pawlyta, M.; Chrusciel, A.; Wyrwol, B. The Use of UVC Irradiation to Sterilize Filtering Facepiece Masks Limiting Airborne Cross-Infection. Int. J. Environ. Res. Public Health 2020, 17, 7396. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Roy, P.; Das, S.; Paul, M.K. A hybrid model integrating warm heat and ultraviolet germicidal irradiation might efficiently disinfect respirators and personal protective equipment. Am. J. Infect. Control 2021, 49, 309–318. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, Z.; Lanzarini-Lopes, M.; Sinha, S.; Rho, H.; Herckes, P.; Westerhoff, P. Germicidal Ultraviolet Light Does Not Damage or Impede Performance of N95 Masks Upon Multiple Uses. Environ. Sci. Technol. Lett. 2020, 7, 600–605. [Google Scholar] [CrossRef]
- Sears, A.P.; Ohayon, J.; Shutov, A.D.; Pettigrew, R.I. Modeling-based UV-C decontamination of N95 masks optimized to avoid undertreatment. medRxiv 2020, 1–8. [Google Scholar] [CrossRef]
- Baluja, A.; Arines, J.; Vilanova, R.; Cortiñas, J.; Bao-Varela, C.; Flores-Arias, M.T. UV light dosage distribution over irregular respirator surfaces. Methods and implications for safety. J. Occup. Environ. Hyg. 2020, 17, 1–8. [Google Scholar] [CrossRef]
- Bergman, M.S.; Viscusi, D.J.; Heimbuch, B.K.; Wander, J.D.; Sambol, A.R.; Shaffer, R.E. Evaluation of Multiple (3-Cycle) Decontamination Processing for Filtering Facepiece Respirators. J. Eng. Fibers Fabr. 2010, 5, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Bergman, M.S.; Viscusi, D.J.; Palmiero, A.J.; Powell, J.B.; Shaffer, R.E. Impact of three cycles of decontamination treatments on filtering facepiece respirator fit. J. Int. Soc. Respir. Prot. 2011, 28, 48–59. [Google Scholar]
- Gilbert, R.M.; Donzanti, M.J.; Minahan, D.J.; Shirazi, J.; Hatem, C.L.; Hayward-Piatkovskyi, B.; Dang, A.M.; Nelson, K.M.; Bothi, K.L.; Gleghorn, J.P. Mask Reuse in the COVID-19 Pandemic: Creating an Inexpensive and Scalable Ultraviolet System for Filtering Facepiece Respirator Decontamination. Glob. Health Sci. Pract. 2020, 8, 582–595. [Google Scholar] [CrossRef] [PubMed]
- Golladay, G.J.; Leslie, K.A.; Zuelzer, W.A.; Cassano, A.D.; Plauny, J.J.; Daniels, F.E.; Bearman, G.; Kates, S.L. Rationale and process for N95 respirator sanitation and re-use in the COVID-19 pandemic. Infect. Control Hosp. Epidemiol. 2021, 1–20. [Google Scholar] [CrossRef]
- Venkatesh, R.; Gurnani, B.; Kaur, K.; Vedachalam, R.; Gubert, J. Innovative application of ultraviolet rays and hydrogen peroxide vapor for decontamination of respirators during COVID-19 pandemic- An experience from a tertiary eye care hospital. Indian J. Ophthalmol. 2020, 68, 1714–1715. [Google Scholar] [CrossRef]
- Yujie, M.; Rae, Z.; Kurt, R.; Kelly, B. Effect of Ultraviolet C Disinfection Treatment on the Nanomechanical and Topographic Properties of N95 Respirator Filtration Microfibers. MRS Adv. 2020, 5, 2863–2872. [Google Scholar] [CrossRef] [PubMed]
- Ou, Q.; Pei, C.; Kim, S.C.; Abell, E.; Pui, D.Y. Evaluation of decontamination methods for commercial and alternative respirator and mask materials—View from filtration aspect. J. Aerosol Sci. 2020, 150, 105609. [Google Scholar] [CrossRef] [PubMed]
- Peltier, R.E.; Wang, J.; Hollenbeck, B.L.; Lanza, J.; Furtado, R.M.; Cyr, J.; Ellison, R.T.; Kobayashi, K.J. Addressing decontaminated respirators: Some methods appear to damage mask integrity and protective function. Infect. Control Hosp. Epidemiol. 2020, 41, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Rohit, A.; Rajasekaran, S.; Shenoy, S.; Rai, S.; Iddya, K.; Dorairajan, S.K. Reprocessing of N95 masks: Experience from a resource-limited setting in India. Int. J. Infect. Dis. 2021, 104, 41–44. [Google Scholar] [CrossRef]
- Salter, W.B.; Kinney, K.; Wallace, W.H.; Lumley, A.E.; Heimbuch, B.K.; Wander, J.D. Analysis of Residual Chemicals on Filtering Facepiece Respirators After Decontamination. J. Occup. Environ. Hyg. 2010, 7, 437–445. [Google Scholar] [CrossRef] [PubMed]
- Viscusi, D.J.; King, W.P.; Shaffer, R.E. Effect of decontamination on the filtration efficiency of two filtering facepiece respirator models. J. Int. Soc. Respir. Prot. 2007, 24, 93–107. [Google Scholar]
- Viscusi, D.J.; Bergman, M.S.; Eimer, B.C.; Shaffer, R.E. Evaluation of Five Decontamination Methods for Filtering Facepiece Respirators. Ann. Occup. Hyg. 2009, 53, 815–827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Viscusi, D.J.; Bergman, M.S.; Novak, D.A.; Faulkner, K.A.; Palmiero, A.; Powell, J.; Shaffer, R.E. Impact of Three Biological Decontamination Methods on Filtering Facepiece Respirator Fit, Odor, Comfort, and Donning Ease. J. Occup. Environ. Hyg. 2011, 8, 426–436. [Google Scholar] [CrossRef] [PubMed]
- Card, K.J.; Crozier, D.; Dhawan, A.; Dinh, M.; Dolson, E.; Farrokhian, N.; Gopalakrishnan, V.; Ho, E.; Jagdish, T.; King, E.S.; et al. UV Sterilization of Personal Protective Equipment with Idle Laboratory Biosafety Cabinets during the Covid-19 Pandemic. medRxiv 2020, 1–18. [Google Scholar] [CrossRef] [Green Version]
- Chen, P.Z.; Ngan, A.; Manson, N.; Maynes, J.T.; Borschel, G.H.; Rotstein, O.D.; Gu, F.X. Transmission of aerosols through pristine and reprocessed N95 respirators. medRxiv 2020, 1–9. [Google Scholar] [CrossRef]
- Lowe, J.J.; Paladino, K.D.; Farke, J.D.; Boulter, C.K.; Emodi, M.; Hankins, R.; Hinkle, L.; Micheels, T.; Schwedhelm, S.; Vasa, A.; et al. N95 Filtering Facepiece Respirator Ultraviolet Germicidal Irradiation (UVGI) Process for De-contamination and Reuse; Nebraska Medicine: Nebraska Medical Center, Omaha, NE, USA, 2020. [Google Scholar]
- Price, A.; Cui, Y.; Liao, L.; Xiao, W.; Yu, X.; Wang, H.; Zhao, M.; Wang, Q.; Chu, S.; Chu, L. Is the fit of N95 facial masks effected by disinfection? A study of heat and UV disinfection methods using the OSHA protocol fit test. medRxiv 2020. [Google Scholar] [CrossRef]
- Borro, L.; Raponi, M.; Del Fatore, A.; Zanini, F.; di Lillo, F.; Contillo, A.; Di Piazza, E.; Bordonaro, V.; Tozzi, A.E.; Secinaro, A. Reusability of P3 facial filter in pandemic emergency: A 3D Analysis of a filter microstructure with X-Ray micro-tomography images after dry heat and UV sterilization procedures. Res. Sq. 2020. [Google Scholar] [CrossRef]
| Sample | Biological Indicators | Nº of Lamps | UV-C Total Dose (J/cm2) | Lamp Power (W) | Exposure to UV-C (min) | Sterilization Cycles | Log Reduction | Toxic Byproduct | Filtration Powers | Changes in Integrity or Fit | Source |
|---|---|---|---|---|---|---|---|---|---|---|---|
| VIRUSES | |||||||||||
| Surgical Masks/ Procedure Masks/FFP1 | |||||||||||
| Surgical masks $ | PRCV strain 91V44 | 4 | 2.6 | 5.5 | 2 | 1 | Yes, >5 | No | No | No | [34] |
| Surgical mask | H1N1 Influenza A virus | 2 | 1.35 | 30 | 15 | Up to 30 | Yes, ≥4 | No | Yes, little or no effect. | Yes, no physical changes after 30 cycles. | [35] |
| Surgical mask $ | Infectious porcine respiratory coronavirus (PRCV strain 91V44) and murine norovirus (MuNoV line RAW264.7 ATCC TIB-71) | 4 | 2.6 & | 5.5 | 2 | Up to 5 | Yes, 5.37 (PRCV) and 4.65 (MuNoV) | No | Yes, little or no effect. | Yes, slightly decreased airflow resistance. | [36] |
| FFP2/KN95/N95 FFR | |||||||||||
| N95 $ | H1N1 influenza (VR-1469) covered with artificial saliva or skin oil | 8 | 1 | 0.39 | 1, 10 # | 1 | Yes, ≥3 in 12/15 FFRs and 7/15 straps for both soiling conditions | No | No | No | [1] |
| N95 | MS2 coliphage | 1 | 38 to 4707 | 40 | 2 to 266 | - | Yes, >3 (after 1000 J of irradiation) | No | No | No | [6] |
| N95 | MS2 bacteriophages and Phi6 | 2 | - | - | 22 (each cycle) or 31 (once extended) | Up to 3 | Yes, ≥2.1 (MS2) single cycle. >6 (three consecutive cycles or extended) | No | No | Yes, no physical changes after three cycles. | [37] |
| N95 | Hcov-19 ncov-WA1-2020 (MN985325.1) | 1 | 0.33 to 1.98 | - | Multiple (10, 30, and 60) | Up to 3 | Yes, >3 | No | Yes, little or no effect. | Yes, it reached the minimum at fit test after three cycles. | [38] |
| N95 | RIX4414 strain of the human rotavirus G1P[8] Wa strain | 1 | - | - | 15 & | Up to 5 | Yes, “the rotaviral RNA was detected on both decontamination methods, while the back of N95 respirators, the rotaviral RNA was undetected.” (p. 50) | No | Yes, little or no effect. | No | [39] |
| N95 | H1N1 influenza (VR-1469) using droplet and aerosol applications | 1 | 1.8 | 80 | 15 | 1 | Yes, >4 (all models both applications) | No | No | Yes, no physical changes. | [40] |
| N95 $ | Escherichia virus MS2. | 8 | >2 | - | 1 | 1 | Yes, >3 | No | No | No | [41] |
| N95 $ | Escherichia virus MS2 (MS2), Pseudomonas virus phi6 (Phi6) | 8 | >2 | - | 1 | 1 | Yes, >2 (MS2 and Phi6) | No | No | No | [41] |
| N95 | Vesicular stomatitis virus | Up to 1.12 | - | - | Up to 5 | Yes, >4 | No | Yes, little or no effect after ten cycles. | Yes, effect after ten cycles. | [42] | |
| N95 | Influenza A/H5N1 (VNH5N1) | 2 | 18,000 | - | 15 | 1 | Yes, >4 (all models) | No | Yes, little or no effect. | No | [43] |
| KN95 $ | PRCV strain 91V44 | 4 | 5.2 | 5.5 | 4 | 1 | Yes, >4 | No | No | No | [34] |
| N95 | Lentivirus bearing a GFP reporter (a surrogate for SARS-CoV-2) | - | 1.8 | - | <30 (7 white cycle, 10.5 colored cycle, 12 heat) | 3 | Yes, ~5 (UV-C alone) | Yes, minimal ozone accumulation | Yes, little or no effect. | Yes, no physical changes. | [44] * |
| N95 | SARS-CoV-2 (USA-WA1/202) | - | - | - | Up to 5 | 1 | Yes, >4.79 | No | No | No | [45] |
| N95 | Swine coronavirus (PEDV strain CO2013) | - | 0.36 to 2.52 & | 25 | Multiple (1, 3, 5, 7) & | Multiple | Yes, 4 | No | No | No | [46] |
| N95 | Clinical samples of SARS-CoV-2 | 1 | - | 30 | - | 1 | Yes, it depended on the model | No | No | Yes, internal degradation, producing particulate. | [47] |
| N95 | Staphylococcal bacteriophages (vb_hsa_2002 and P66 phages) | 10 | Multiple | - | 4 | 1 | Yes, >3 | Yes, minimal ozone concentration | Yes, little or no effect. (Effect caused by wearing) | Yes, no physical changes even in dosage corresponding to 50 cycles. | [48] * |
| N95 (one model with a hydrophilic outer layer and another with a hydrophobic outer layer) | MS2 bacteriophage (ATCC 15597-B1) (multiple deposition methods: droplets, vaporized, and aerosolized) | 1 | ~1 | 40 | 5 | 1 | Yes, >5 (for all models and methods). | Yes, no toxic byproduct left. | Yes, little or no effect. | Yes, no physical changes. | [49] |
| N95 | MS2 coliphage | 1 | 4.32 | 40 | Up to 300 | 1 | Yes, >3 after three hours. No virus after five hours at ~7.20 J/cm2. | No | No | No | [50] |
| N95/KN95 | MS2, Phi6, influenza A virus, murine hepatitis virus | 1 | - | - | 15 | 1 | Yes, but <2 (MS2, Phi6, influenza A, MHV) (only UV-PX). | No | Yes, little or no effect. | Yes, no physical changes. | [51] * |
| N95 | MS2 bacteriophage (multiple deposition methods and humidity levels) | 1 | - | 4 | Up to 240 | Multiple | Yes, multiple results depending on the relative humidity of the coupon face and the deposition method (the highest was 5.8) | No | No | No | [52] |
| N95 | Swine coronavirus (PEDV) | 1 | - | 4 | Multiple (10, 15, and 20), 1 # | 1 | Yes, “it is likely that 10-min UV-C is sufficient for the inactivation of the virus” (p. 06) | No | No | Yes, no physical changes. | [53] |
| N95 | Hcov-19 ncov-WA1-2020 (MN985325.1) | 1 | 0.33 to 1.98 | - | Multiple (10, 30, and 60) | Up to 3 | Yes, >3 | No | Yes, little or no effect after three cycles. | Yes, minimum at fit test after three cycles. | [54] |
| N95 $ | SARS-CoV-2 | UV LEDs | 0.3 to 0.6 | - | Multiple (0, 5, and 10) | Up to 3 | Yes, >3 (in one model) | No | No | No | [55] |
| KN95 $ | Infectious porcine respiratory coronavirus (PRCV strain 91V44) and murine norovirus (MuNoV line RAW264.7 ATCC TIB-71) | 4 | 2.6 & | 5.5 | 2 | 5 | Yes, 4.48 (PRCV) and 4.33 (MuNoV) | No | Yes, little or no effect. | Yes, no physical changes. | [36] |
| N95 $ | SARS-CoV-2 (USA-WA1/2020 NR-52281) | - | 1.5 & | - | 1 to 1.16 & | 1 | Yes, it depended on the model | No | No | No | [56] |
| N95 | SARS-CoV-2 (USA-WA1/202, bei resource NR52281) | 2 | 1.5 | - | 0 to 2.73 | 1 | Yes, 3.5 | No | No | No | [57] |
| N95 | Human coronavirus NL63 | 1 | - | - | 15 | 1 | Yes, >3 | No | No | No | [58] |
| BACTERIA | |||||||||||
| Surgical Masks/ Procedure Masks/FFP1 | |||||||||||
| FFP1 and surgical mask | E. Coli (K12) and B. Subtilis (B 4056) | 1 | Up to 0.378 | - | Multiple (5, 10, 15) | 1 | - | No | Yes, little or no effect. | No | [59] |
| surgical mask | S. Aureus | 2 | 1.35 | 30 | 15 | Up to 30 | Yes, ≥4 | No | Yes, little or no effect. | Yes, no physical changes after 30 cycles. | [35] |
| FFP1 and surgical mask | S. Aureus | 24 | 2.7 | 95 | 30 | Up to 3 | Yes, ≥8 | No | No | No | [60] |
| surgical mask | S. Aureus | 1 | - | 20 | 5 & | Up to 3 | Yes, 4 | No | Yes, little or no effect after three cycles. | Yes, no physical changes after a 90-min exposure. | [61] |
| FFP2/KN95/N95 FFR | |||||||||||
| N95 | Methicillin-resistant S. Aureus (MRSA) | 2 | - | - | 22 (each cycle) or 31 (once extended) | Up to 3 | Yes, >6 (single cycle, consecutive cycles, and extended) | No | No | Yes, no physical changes after three cycles. | [37] |
| FFP2 | E. Coli (K12) and B. Subtilis (B 4056) | 1 | Up to 0.378 | Multiple (5, 10, 15) | 1 | Yes, “No surviving bacterium was observed after UVI treatment for 5 min or longer” (p. 13166) | No | Yes, little or no effect even when 20 J/cm2. | No | [59] | |
| N95 (5 layers: coverweb, stiffener, 1st and 2nd filter layers, innerweb)/KF94 (3 layers: coverweb, filter web, inner web) | E. Coli (KCTC 1039) | 1 | - | 10 | 60 & | 1 | Yes, <2 | Yes, peaks of C–O–C and O–H bending | Yes, little or no effect. | Yes, no physical changes | [62] |
| N95 $ | Methicillin-resistant S. Aureus (MRSA) and C. Difficile | 8 | >2 | - | Up to 3 | 1 | Yes, >5 (MRSA), <3 (C. difficile) | No | No | No | [41] |
| N95 | B. Subtilis (CCRC 12145) | 1 | - | 6 | Multiple (1, 2, 5, 10, 20) & | 1 | Yes, “no colony was recovered after exposure to UVC for as little as five minutes” (p. 757) | No | No | No | [63] |
| N95 | S. Epidermis, P. Aeruginosa, and G. Stearothermophilus | - | 1.8 | - | <30 (7 white cycle, 10.5 colored cycle, 12 heat) | 3 | Yes, 6 (S. Epidermis and P. Aeruginosa) and > 6 (G. Stearothermophilus) just using UV-C | Yes, minimal ozone accumulation | Yes, little or no effect. | Yes, no physical changes | [44] * |
| N95 | B. Pumilus PM-106 (as a surrogate for SARS-CoV-2) | Variable | ≥1 | 30 (nonozone) | 5, 10 # | Up to 5 | Yes, 6 | Yes, minimal ozone concentration. | Yes, little or no effect. (one model) | Yes, no physical changes through five cycles. (One model) | [64] |
| N95/KN95 | E. Coli, S. Aureus, and G. Stearothermophilus | 1 | - | - | 15 | 1 | Yes, but <1 (S. aureus) (UV-PX alone). | No | Yes, little or no effect. | Yes, no physical changes. | [51] * |
| N95/KN95 | S. Aureus | 24 | 2.7 | 95 | 30 | Up to 3 | Yes, ≥7 | No | No | No | [60] |
| FFP3/KN98/N98 FFR | |||||||||||
| FFP3 | E. Coli (K12) and B. Subtilis (B 4056) | 1 | Up to 0.378 | - | Multiple (5, 10, 15) | 1 | - | No | Yes, little or no effect. | No | [59] |
| Others | |||||||||||
| One mask (with HEPA filter) | B. Atrophaeus (ATCC9372) | 10 | 1 | 17 | 15 | Up to 3 | Yes, “UVC radiation eliminates pathogens in all layers ofthe HEPA filter.” (p. 13) | No | No | Yes, no physical changes. | [65] |
| NO BIOLOGICAL INDICATORS/OTHER BIOLOGICAL INDICATORS | |||||||||||
| Surgical Masks/ Procedure Masks/FFP1 | |||||||||||
| Surgical mask | - | 1 | ≥1 | 0.017 | 1 | 1 | Yes, >5.5 (in 52 min, simulation) | No | No | No | [66] * |
| Surgical mask (two outers of cellulose acetate and interior of polypropylene) | - | 4 | 1 to 10 | 120 | ~2 | - | No | Yes, no toxic byproduct left. | Yes, little or no effect. | Yes, no physical changes. | [67] |
| surgical mask | - | 2 | 2.7 | - | 5 | - | No | No | No | No | [68] |
| FFP2/KN95/N95 FFR | |||||||||||
| N95 $ | - | 2 | 120 to 950 | 15 | - | 1 | No | No | Yes, efficiency reduction of 1.25% in higher doses. | No | [7] |
| N95/KN95/KF94 $ | - | 1 | ~3.6 | 8 | 30, 10 # | 10 | No | No | No | Yes, no physical changes. | [33] |
| FFP2 | - | 3 | 0.3 to 3 | 4.9 | 0.183 to 100 | - | No | No | No | No | [69] |
| N95 | - | 1 | ≥1 | 0.017 | 1 | 1 | Yes, >5.5 (in 52 min, simulation) | No | No | No | [66] * |
| N95/FFP2 | - | 1 | - | 40 | 45 | 1 | No | Yes, no odor. | No | Yes, no physical changes. | [70] |
| N95 | - | 1 | 3.24 (1.62 &) | 40 | 15 | Up to 3 | No | Yes, no odor. | No | Yes, no physical changes. | [71] |
| N95 | - | Variable | 0.3 | - | 19.4 & | 1 | No | No | No | No | [72] |
| N95 (in different sizes) | - | >1 | - | - | 12.5 & | 2 | No | No | Yes, little or no effect. | Yes, no physical changes. | [73] |
| N95 | - | 1 | 0.06 | - | 15 to 20 | Up to 5 | No | No | No | No | [74] * |
| N95 | - | 2 | Multiple (1, 7, 13, 19, 31) | 38 | Multiple (5, 35, 65, 95, 155) | Up to 5 | No | No | Yes, it presented a decrease in fiber filtration power. | Yes, it showed degradation with the increase in dosage. | [75] |
| N95 | - | - | ~1 | - | 5 | 10 | No | No | Yes, little or no effect up to 10 cycles. | No | [76] ** |
| N95 | - | - | - | - | 10 & | 1 (equivalent to 10×) | No | No | Yes, some decrease after the ninth cycle. | No | [77] |
| N95 | - | 8 | - | 18 | 10 | 1 | No | No | No | Yes, it showed degradation after reprocessing, but levels are dependent on the model. | [78] |
| N95 | - | - | 2.7 | 8 | 60 | - | No | Yes, unique peaks, but related to the n-pentane (solvent). | No | No | [79] |
| N95 | - | 2 | - | 40 | Up to 480 (240 &) | 1 | No | No | Yes, little or no effect. | Yes, no physical changes. | [80] |
| N95 | - | 1 | 0.176 to 0.181 & | 40 | 30 (15 &) | 1 | No | No | Yes, little or no effect. | Yes, no physical changes. | [81] |
| N953 | - | 1 | - | 40 | 30 (15 &), Overnight # | Up to 5 | No | Yes, no odor. | Yes, little or no effect. | Yes, no physical changes. | [82] |
| N95/KN95 | fungus Aspergillus niger | 1 | - | - | 15 | 1 | Yes, but <0.3 (UV-PX alone). | No | Yes, little or no effect. | Yes, no physical changes. | [51] * |
| N95 (One model with the second layer: polyester, while the other possesses a plastic-mesh in the outer layer) | - | 4 | 1 to 10 | 120 | ~2 | - | No | Yes, no toxic byproduct left. | Yes, little or no effect. | Yes, no physical changes. | [67] |
| N95 | - | 1 | 1 | 0.0001 | 62 to 258 & | 1 | No | No | No | No | [83] |
| N95 | - | 4 | ≥1 | - | 4 | Multiple (1, 3, 5, and 10) | No | No | Yes, little or no effect (<1.5% at 0.3 μm). | Yes, it “induced slight dose-dependent photochemical damage” (p. 03) after three cycles (p. 30). | [84] |
| N95 | - | 16 | 0.18 to 1.2 | 0.016 | 5 | Up to 5. | No | No | No | No | [85] |
| N95/KN95 $ | - | - | - | 8 | 30, 10 # | Up to 10 | No | No | No | Yes, effect after ten cycles. | [86] |
| N95 | - | 2 | 2.7 | - | 5 | - | No | No | No | No | [68] |
| FFP3/KN98/N98 FFR | |||||||||||
| FFP3 | - | 3 | 0.3 to 3 | 4.9 | 0.183 to 100 | - | No | No | No | No | [69] |
| P3 | - | 1 | - | 40 | 120 | 1 | No | No | Yes, little or no effect. | Yes, no physical changes. | [87] |
| Others | |||||||||||
| Meltblown fabric (20 g/m2) | - | 1 | ~3.6 | 8 | 30, 10 # | 10 | No | No | Yes, reduction to 93% after 20 cycles. | No | [33] |
| P100 | - | 2 | - | 40 | Up to 480 (240 &) | 1 | No | No | Yes, little or no effect, but its results were more variable when the exposure period increased. | Yes, no physical changes. | [80] |
| P100 | - | 1 | 0.176 to 0.181 & | 40 | 30 (15 &) | 1 | No | No | Yes, little or no effect. | Yes, no physical changes. | [81] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nicolau, T.; Filho, N.G.; Zille, A. Ultraviolet-C as a Viable Reprocessing Method for Disposable Masks and Filtering Facepiece Respirators. Polymers 2021, 13, 801. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050801
Nicolau T, Filho NG, Zille A. Ultraviolet-C as a Viable Reprocessing Method for Disposable Masks and Filtering Facepiece Respirators. Polymers. 2021; 13(5):801. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050801
Chicago/Turabian StyleNicolau, Talita, Núbio Gomes Filho, and Andrea Zille. 2021. "Ultraviolet-C as a Viable Reprocessing Method for Disposable Masks and Filtering Facepiece Respirators" Polymers 13, no. 5: 801. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13050801