Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials
Abstract
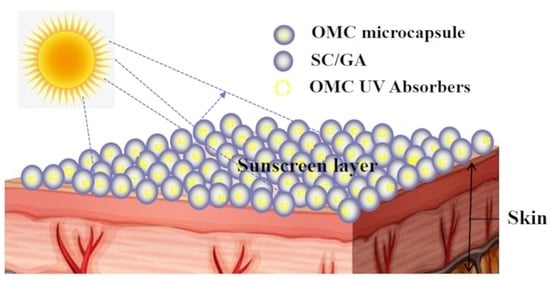
:1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
2.2. Selection of Wall Materials
2.3. Preparation of OMC Microcapsules
2.4. Experimental Designs of OMC Microcapsules
2.5. Characterization of OMC Microcapsules
2.6. Release of OMC from the OMC Microcapsules
2.7. UV Absorption Effect of the OMC Microcapsules
2.8. Test of Sun Protection Factor (SPF) Value
2.9. Statistical Analysis
3. Results and Discussion
3.1. Screening of Natural Polymer Wall Materials
3.2. Preparation of the OMC Microcapsules
3.3. Characterization of the OMC Microcapsules
3.4. Release Behavior of the OMC Microcapsules
3.5. UV Absorption Effect of the OMC Microcapsules
3.6. Effect of Microencapsulation on SPF of Sunscreen
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV filters to protect us: Part 1: Changing regulations and choices for optimal sun protection. Int. J. Women’s Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, S.; Sherje, A.P. Development of Resveratrol and Green Tea Sunscreen Formulation for Combined Photoprotective and Antioxidant Properties. J. Drug Deliv. Sci. Technol. 2020, 60, 102000. [Google Scholar] [CrossRef]
- Guroji, P.; Qayyum, S.; Kim, T.; Janjetovic, Z.; Athar, M.; Slominski, A. 638 Cyp11a1 derived secosteroid, 20(OH)d3 as a novel therapeutic agent for the prevention and treatment of uvb induced skin cancer. J. Investig. Dermatol. 2020, 140, S86. [Google Scholar] [CrossRef]
- Horsham, C.; Ford, H.; Hacker, E. Promoting sunscreen use in adolescents playing outdoor sports using UV detection stick-ers. Prev. Med. Rep. 2020, 19, 101166. [Google Scholar] [CrossRef] [PubMed]
- Daneluti, A.L.M.; Neto, F.M.; Ruscinc, N.; Lopes, I.; Velasco, M.V.R.; Matos, J.D.R.; Baby, A.R.; Kalia, Y.N. Using ordered mesoporous silica SBA-15 to limit cutaneous penetration and transdermal permeation of organic UV filters. Int. J. Pharm. 2019, 570, 118633. [Google Scholar] [CrossRef] [PubMed]
- He, H.; Li, A.; Li, S.; Tang, J.; Li, L.; Xiong, L. Natural components in sunscreens: Topical formulations with sun protection factor (SPF). Biomed. Pharmacother. 2021, 134, 111161. [Google Scholar] [CrossRef] [PubMed]
- Slomberg, D.L.; Catalano, R.; Bartolomei, V.; Labille, J. Release and fate of nanoparticulate TiO2 UV filters from sunscreen: Effects of particle coating and formulation type. Environ. Pollut. 2021, 271, 116263. [Google Scholar] [CrossRef] [PubMed]
- Keyes, E.; Werth, V.P.; Brod, B. Potential allergenicity of commonly sold high SPF broad spectrum sunscreens in the United States; from the perspective of patients with autoimmune skin disease. Int. J. Women’s Dermatol. 2019, 5, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Volpe, V.; Nascimento, D.S.; Insausti, M.; Grünhut, M. Octyl p-methoxycinnamate loaded microemulsion based on Ocimum basilicum essential oil. Characterization and analytical studies for potential cosmetic applications. Colloids Surf. A Physicochem. Eng. Asp. 2018, 546, 285–292. [Google Scholar] [CrossRef]
- Mota, M.D.; da Morte, A.N.; Silva, L.C.R.C.E.; Chinalia, F.A. Sunscreen protection factor enhancement through supple-mentation with Rambutan (Nephelium lappaceum L) ethanolic extract. J. Photochem. Photobiol. B 2020, 205, 111837. [Google Scholar] [CrossRef]
- Nataraj, B.; Maharajan, K.; Hemalatha, D.; Rangasamy, B.; Arul, N.; Ramesh, M. Comparative toxicity of UV-filter Octyl meth-oxycinnamate and its photoproducts on zebrafish development. Sci. Total Environ. 2020, 718, 134546. [Google Scholar] [CrossRef]
- Cahova, J.; Blahova, J.; Marsalek, P.; Doubkova, V.; Franc, A.; Garajová, M.; Tichy, F.; Mares, J.; Svobodova, Z. The biological activity of the organic UV filter ethylhexyl methoxycinnamate in rainbow trout (Oncorhynchus mykiss). Sci. Total Environ. 2021, 774, 145570. [Google Scholar] [CrossRef] [PubMed]
- Yamada, M.; Mohammed, Y.; Prow, T.W. Advances and controversies in studying sunscreen delivery and toxicity. Adv. Drug Deliv. Rev. 2020, 153, 72–86. [Google Scholar] [CrossRef] [PubMed]
- Castro, N.R.; Pinto, C.d.S.C.; de Campos, V.E.B.; Cardoso, V.; Vermelho, A.B.; Santos, E.P.D.; Mansur, C.R.E. Development of hybrid vesicular nanosystems composed of lipids and chitosan for octyl methoxycinnamate encapsulation. Colloid. Surf. A 2021, 608, 125476. [Google Scholar] [CrossRef]
- Eriksson, V.; Trojer, M.A.; Vavra, S.; Hulander, M.; Nordstierna, L. Formulation of polyphthalaldehyde microcap-sules for immediate UV-light triggered release. J. Colloid Interface Sci. 2020, 579, 645–653. [Google Scholar] [CrossRef] [PubMed]
- Budinčić, J.M.; Petrović, L.; Đekić, L.; Fraj, J.; Bučko, S.; Katona, J.; Spasojević, L. Study of vitamin E microencapsulation and controlled release from chitosan/sodium lauryl ether sulfate microcapsules. Carbohydr. Polym. 2021, 251, 116988. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Fang, K.; He, W.; Li, K.; Jiang, Y.; Li, J. Evaluation of chitosan-ferulic acid microcapsules for sustained drug delivery: Synthesis, characterizations, and release kinetics in vitro. J. Mol. Struct. 2021, 1227, 129353. [Google Scholar] [CrossRef]
- Ferreira, S.; Nicoletti, V.R. Microencapsulation of ginger oil by complex coacervation using atomization: Effects of polymer ratio and wall material concentration. J. Food Eng. 2021, 291, 110214. [Google Scholar] [CrossRef]
- Vargas, S.A.; Delgado-Macuil, R.J.; Ruiz-Espinosa, H.; Rojas-López, M.; Amador-Espejo, G.G. High-intensity ultrasound pre-treatment influence on whey protein isolate and its use on complex coacervation with kappa carrageenan: Evaluation of se-lected functional properties. Ultrason Sonochem. 2021, 70, 105340. [Google Scholar] [CrossRef] [PubMed]
- González-Monje, P.; García, A.A.; Ruiz-Molina, D.; Roscini, C. Encapsulation and sedimentation of nanomaterials through complex coacervation. J. Colloid Interface Sci. 2021, 589, 500–510. [Google Scholar] [CrossRef]
- Boyd, B.J. Characterisation of drug release from cubosomes using the pressure ultrafiltration method. Int. J. Pharm. 2003, 260, 239–247. [Google Scholar] [CrossRef]
- Schwarzl, R.; Du, F.; Haag, R.; Netz, R.R. General method for the quantification of drug loading and release kinetics of nanocarriers. Eur. J. Pharm. Biopharm. 2017, 116, 131–137. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanha, N.; Regenstein, J.M.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Properties and kinetics of the in vitro release of an-thocyanin-rich microcapsules produced through spray and freeze-drying complex coacervated double emulsions. Food Chem. 2021, 340, 127950. [Google Scholar] [CrossRef] [PubMed]
- Azhdari, E.; Emami, A.; Ferreira, J. Drug release from a surface erosion biodegradable viscoelastic polymeric platform: Analysis and numerical simulation. Comput. Math. Appl. 2020, 80, 3004–3026. [Google Scholar] [CrossRef]
- Tomazelli, L.C.; Ramos, M.M.D.A.; Sauce, R.; Cândido, T.M.; Sarruf, F.D.; Pinto, C.A.S.D.O.; de Oliveira, C.A.; Rosado, C.; Velasco, M.V.R.; Baby, A.R. SPF enhancement provided by rutin in a multifunctional sunscreen. Int. J. Pharm. 2018, 552, 401–406. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-R.; Chang, Y.H. Microencapsulation of gallic acid through the complex of whey protein concentrate-pectic polysac-charide extracted from Ulmus davidiana. Food Hydrocoll. 2018, 85, 222–228. [Google Scholar] [CrossRef]
- Damiani, E.; Puglia, C. Nanocarriers and Microcarriers for Enhancing the UV Protection of Sunscreens: An Overview. J. Pharm. Sci. 2019, 108, 3769–3780. [Google Scholar] [CrossRef] [PubMed]
- Romanhole, R.C.; Fava, A.L.M.; Tundisi, L.L.; de Macedo, L.M.; dos Santos, É.M.; Ataide, J.A.; Mazzola, P.G. Unplanned absorption of sunscreen ingredients: Impact of formulation and evaluation methods. Int. J. Pharm. 2020, 591, 120013. [Google Scholar] [CrossRef]
- Marques da Silva, T.; Lopes, E.J.; Codevilla, C.F.; Cichoski, A.J.; Flores, É.M.D.M.; Motta, M.H.; da Silva, C.D.B.; Grosso, C.R.F.; de Menezes, C.R. Development and characterization of microcapsules containing Bifidobacterium Bb-12 produced by complex coacervation followed by freeze drying. LWT 2018, 90, 412–417. [Google Scholar] [CrossRef]
- Frelichowska, J.; Bolzinger, M.-A.; Pelletier, J.; Valour, J.-P.; Chevalier, Y. Topical delivery of lipophilic drugs from o/w Pick-ering emulsions. Int. J. Pharmaceut. 2009, 371, 56–63. [Google Scholar] [CrossRef]
- Da Cruz, M.C.R.; Dagostin, J.L.A.; Perussello, C.A.; Masson, M.L. Assessment of physicochemical characteristics, thermal stability and release profile of ascorbic acid microcapsules obtained by complex coacervation. Food Hydrocoll. 2019, 87, 71–82. [Google Scholar] [CrossRef]
- Li, H.; Feng, Y.; Cui, Y.; Ma, Y.; Zheng, Z.; Qian, B.; Wang, H.; Semenov, A.; Shchukin, D. Polyurea/polyaniline hybrid shell mi-crocapsules loaded with isophorone diisocyanate for synergetic self-healing coatings. Prog. Org. Coat. 2020, 145, 105684. [Google Scholar] [CrossRef]
- Qian, B.; Zheng, Z.; Liu, C.; Li, M.; D’Sa, R.A.; Li, H.; Graham, M.; Michailidis, M.; Kantserev, P.; Vinokurov, V.; et al. Mi-crocapsules Prepared via Pickering Emulsion Polymerization for Multifunctional Coatings. Prog. Org. Coat. 2020, 147, 105785. [Google Scholar] [CrossRef]
- Su, J.-F.; Schlangen, E. Synthesis and physicochemical properties of high compact microcapsules containing rejuvenator ap-plied in asphalt. Chem. Eng. J. 2012, 198–199, 289–300. [Google Scholar] [CrossRef]
- Kanha, N.; Surawang, S.; Pitchakarn, P.; Laokuldilok, T. Microencapsulation of copigmented anthocyanins using double emulsion followed by complex coacervation: Preparation, characterization and stability. LWT 2020, 133, 110154. [Google Scholar] [CrossRef]
- Andreani, T.; Dias-Ferreira, J.; Fangueiro, J.; Souza, A.; Kiill, C.; Gremião, M.; García, M.; Silva, A.; Souto, E. Formulating octyl methoxycinnamate in hybrid lipid-silica nanoparticles: An innovative approach for UV skin protection. Heliyon 2020, 6, e03831. [Google Scholar] [CrossRef]
- Yang, Y.; Ako-Adounvo, A.-M.; Wang, J.; Zhang, J.; Willett, D.; Yilmaz, H.; Korang-Yeboah, M.; Hsu, H.-J.; Coelho, S.G.; Adah, S.A.; et al. In Vitro Testing of Sunscreens for Dermal Absorption: A Platform for Product Selection for Maximal Usage Clinical Trials. J. Investig. Dermatol. 2020, 140, 2487–2495. [Google Scholar] [CrossRef]
- Gollavilli, H.; Hegde, A.R.; Managuli, R.S.; Bhaskar, K.V.; Dengale, S.J.; Reddy, M.S.; Kalthur, G.; Mutalik, S. Naringin nano-ethosomal novel sunscreen creams: Development and performance evaluation. Colloids Surf. B Biointerfaces 2020, 193, 111122. [Google Scholar] [CrossRef]
- Infante, V.; Campos, P.M.; Calixto, L.; Darvin, M.; Kröger, M.; Schanzer, S.; Lohan, S.; Lademann, J.; Meinke, M. Influence of physical–mechanical properties on SPF in sunscreen formulations on ex vivo and in vivo skin. Int. J. Pharm. 2021, 598, 120262. [Google Scholar] [CrossRef]
- Lin, Y.-C.; Fang, Y.-P.; Hung, C.-F.; Yu, H.-P.; Alalaiwe, A.; Wu, Z.-Y.; Fang, J.-Y. Multifunctional TiO2/SBA-15 mesoporous sili-ca hybrids loaded with organic sunscreens for skin application: The role in photoprotection and pollutant adsorption with reduced sunscreen permeation. Colloids Surf. B 2021, 202, 1111658. [Google Scholar] [CrossRef] [PubMed]














| Stirring Speed | Ratio of Core Material to Wall Material | Curing Agent Content | |
|---|---|---|---|
| Orthogonal | 200 | 1:2 | 1% |
| 400 | 1:1 | 2% | |
| 600 | 2:1 | 3% |
| Components | Mass Fraction (%) |
|---|---|
| Phase A | |
| Cetearyl Alcohol | 2.21 |
| PEG-40 Castor oil | 0.63 |
| Sodium Cetearyl Sulphate | 0.32 |
| Decyl Oleate | 15.00 |
| Ethylhexyl Methoxycinnamate | 3.00 |
| Butyl Methoxydibenzoylmethane | 0.50 |
| Propylparaben | 0.10 |
| Phase B | |
| Water | 53.57 |
| 2-Phenyl-Benzimidazole-5-Sulphonic Acid | 2.78 |
| Sodium Hydroxide (45% solution) | 0.90 |
| Methylparaben | 0.30 |
| Disodium Ethylene Diamine Tetraacetic Acid (EDTA) | 0.10 |
| Phase C | |
| Water | 20.00 |
| Carbomer | 0.30 |
| Sodium Hydroxide (45% solution) | 0.30 |
| Sample | Zeta (mV) | Size (μm) | PDI |
|---|---|---|---|
| S1 | 10.09 ± 0.484 | 15.32 ± 0.457 | 0.348 ± 0.006 |
| S2 | 8.47 ± 0.216 | 14.45 ± 0.289 | 0.432 ± 0.007 |
| S3 | 7.14 ± 0.269 | 13.28 ± 0.305 | 0.332 ± 0.006 |
| S4 | 4.43 ± 0.154 | 12.52 ± 0.401 | 0.323 ± 0.006 |
| S5 | 2.93 ± 0.087 | 11.32 ± 0.232 | 0.301 ± 0.004 |
| S6 | 1.56 ± 0.048 | 10.01 ± 0.012 | 0.202 ± 0.002 |
| S7 | −0.19 ± 0.006 | 8.32 ± 0.175 | 0.322 ± 0.003 |
| S8 | −0.85 ± 0.026 | 7.56 ± 0.173 | 0.345 ± 0.002 |
| S9 | −2.18 ± 0.087 | 5.53 ± 0.143 | 0.366 ± 0.004 |
| Run | Speed (rpm) | OMC to GA/SC Ratio (g/g) | Curing Agent Content (%) | EE (%) |
|---|---|---|---|---|
| 1 | 200 | 1:1 | 1% | 83.3 |
| 2 | 200 | 1:2 | 2% | 32.7 |
| 3 | 200 | 2:1 | 3% | 53.4 |
| 4 | 400 | 1:1 | 2% | 21.3 |
| 5 | 400 | 1:2 | 3% | 38.9 |
| 6 | 400 | 2:1 | 1% | 54.2 |
| 7 | 600 | 1:1 | 3% | 61.2 |
| 8 | 600 | 1:2 | 1% | 63.6 |
| 9 | 600 | 2:1 | 2% | 22.7 |
| K1 | 0.387 | 0.349 | 0.670 | |
| K2 | 0.252 | 0.321 | 0.256 | |
| K3 | 0.288 | 0.256 | 0.145 | |
| R | 0.135 | 0.093 | 0.670 |
| Models | Parameters | |||
|---|---|---|---|---|
| Zero-order | OMC | The OMC Microcapsule | ||
| k | 0.05385 | k | 0.02945 | |
| R2 | 0.5705 | R2 | 0.8034 | |
| First-order | k | 0.003610 | k | 0.00277 |
| R2 | 0.9705 | R2 | 0.9771 | |
| Higuchi | k | 0.6877 | k | 0.5318 |
| R2 | 0.8409 | R2 | 0.8041 | |
| Rigter-Peppas | k | 0.6712 | k | 0.7924 |
| n | 0.4571 | n | 0.5472 | |
| R2 | 0.9812 | R2 | 0.9964 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xu, C.; Zeng, X.; Yang, Z.; Ji, H. Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials. Polymers 2021, 13, 866. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060866
Xu C, Zeng X, Yang Z, Ji H. Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials. Polymers. 2021; 13(6):866. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060866
Chicago/Turabian StyleXu, Chuntao, Xuemin Zeng, Zujin Yang, and Hongbing Ji. 2021. "Sunscreen Enhancement of Octyl Methoxycinnamate Microcapsules by Using Two Biopolymers as Wall Materials" Polymers 13, no. 6: 866. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13060866