Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis of EA
2.2. Procurement and Incorporation of Si Particles in EA
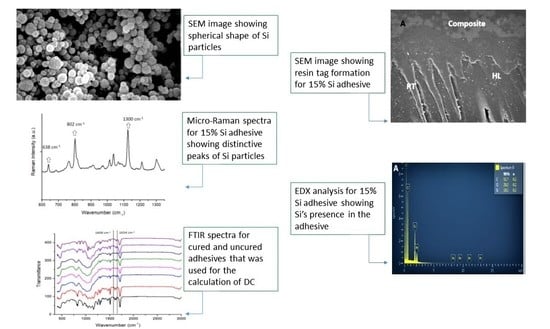
2.3. Characterization of Si Particles
2.4. Preparation of Tooth Samples
2.5. µ TBS Analysis
2.6. Investigation of Bonded Adhesive–Dentin Interface Using SEM Spectroscopy
2.7. Degree of Conversion Analysis
- Caliphatic = 1638 cm−1 absorption peak of cured resin;
- Caromatic = 1607 cm−1 absorption peak of cured resin;
- Ualiphatic = 1638 cm−1 absorption peak of uncured resin;
- Uaromatic = 1607 cm−1 absorption peak of uncured resin.
2.8. Statistical Analysis
3. Results
3.1. SEM-EDX Analysis Results
3.2. Micro-Raman Spectroscopy Results
3.3. µTBS and Failure Mode Analysis Results
3.4. FTIR and DC Analysis Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stewart, C.A.; Hong, J.H.; Hatton, B.D.; Finer, Y. Responsive antimicrobial dental adhesive based on drug-silica co-assembled particles. Acta Biomater. 2018, 76, 283–294. [Google Scholar] [CrossRef]
- Ranjitha, G.; Vikram, R.; Meena, N.; Vijayalakshmi, L.; Murthy, C.S. Clinical efficacy of universal adhesives for the restoration of noncarious cervical lesions: A randomized clinical trial. J. Conserv. Dent. 2020, 23, 227–232. [Google Scholar] [CrossRef]
- Foxton, R.M. Current perspectives on dental adhesion: (2) Concepts for operatively managing carious lesions extending into dentine using bioactive and adhesive direct restorative materials. Jpn. Dent. Sci. Rev. 2020, 56, 208–215. [Google Scholar] [CrossRef] [PubMed]
- Migliau, G. Classification review of dental adhesive systems: From the IV generation to the universal type. Ann. Stomatol. 2017, 8, 1–17. [Google Scholar] [CrossRef]
- Guo, J.; Wang, L.-P.; Zhu, J.; Yang, J.; Zhu, H.-S. Impact of Dentinal Tubule Orientation on Dentin Bond Strength. Curr. Med. Sci. 2018, 38, 721–726. [Google Scholar] [CrossRef]
- Nagarkar, S.; Theis-Mahon, N.; Perdigão, J. Universal dental adhesives: Current status, laboratory testing, and clinical performance. J. Biomed. Mater. Res. Part B Appl. Biomater. 2019, 107, 2121–2131. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharya, M. Polymer Nanocomposites—A Comparison between Carbon Nanotubes, Graphene, and Clay as Nanofillers. Materials 2016, 9, 262. [Google Scholar] [CrossRef]
- Fugolin, A.; Pfeifer, C. New Resins for Dental Composites. J. Dent. Res. 2017, 96, 1085–1091. [Google Scholar] [CrossRef]
- Buchwald, Z.; Sandomierski, M.; Voelkel, A. Calcium-Rich 13X Zeolite as a Filler with Remineralizing Potential for Dental Composites. ACS Biomater. Sci. Eng. 2020, 6, 3843–3854. [Google Scholar] [CrossRef] [PubMed]
- Al-Hamdan, R.S.; Almutairi, B.; Kattan, H.F.; Alsuwailem, N.A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Influence of Hydroxyapatite Nanospheres in Dentin Adhesive on the Dentin Bond Integrity and Degree of Conversion: A Scanning Electron Microscopy (SEM), Raman, Fourier Transform-Infrared (FTIR), and Microtensile Study. Polymers 2020, 12, 2948. [Google Scholar] [CrossRef] [PubMed]
- Alfawaz, Y.F.; Almutairi, B.; Kattan, H.F.; Zafar, M.S.; Farooq, I.; Naseem, M.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Hydroxyapatite Containing Resin Adhesive Enhanced with Graphene Oxide Nano-Particles—An SEM, EDX, Micro-Raman, and Microtensile Bond Strength Study. Polymers 2020, 12, 2978. [Google Scholar] [CrossRef]
- Ramos, N.; Kaizer, M.; Campos, T.; Kim, J.; Zhang, Y.; Melo, R. Silica-Based Infiltrations for Enhanced Zirconia-Resin Interface Toughness. J. Dent. Res. 2019, 98, 423–429. [Google Scholar] [CrossRef]
- Wang, J.; Yu, Q.; Yang, Z. Effect of hydrophobic surface treated fumed silica fillers on a one-bottle etch and rinse model dental adhesive. J. Mater. Sci. Mater. Electron. 2017, 29, 10. [Google Scholar] [CrossRef] [PubMed]
- Alam Moheet, I.; Luddin, N.; Ab Rahman, I.; Kannan, T.P.; Ghani, N.R.N.A.; Masudi, S.M. Modifications of Glass Ionomer Cement Powder by Addition of Recently Fabricated Nano-Fillers and Their Effect on the Properties: A Review. Eur. J. Dent. 2019, 13, 470–477. [Google Scholar] [CrossRef] [Green Version]
- Lührs, A.-K.; Geurtsen, W. The Application of Silicon and Silicates in Dentistry: A Review. Prog. Mol. Subcell. Biol. 2009, 47, 359–380. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Fan, Y.; Zhang, J.-F.; Hagan, J.L.; Xu, X. Novel dental composites reinforced with zirconia–silica ceramic nanofibers. Dent. Mater. 2012, 28, 360–368. [Google Scholar] [CrossRef] [Green Version]
- Bai, X.; Lin, C.; Wang, Y.; Ma, J.; Wang, X.; Yao, X.; Tang, B. Preparation of Zn doped mesoporous silica nanoparticles (Zn-MSNs) for the improvement of mechanical and antibacterial properties of dental resin composites. Dent. Mater. 2020, 36, 794–807. [Google Scholar] [CrossRef] [PubMed]
- Timpe, N.; Fullriede, H.; Borchers, L.; Stiesch, M.; Behrens, P.; Menzel, H. Nanoporous silica nanoparticles with spherical and anisotropic shape as fillers in dental composite materials. BioNanoMaterials 2014, 15, 89–99. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamaguchi, K.; Tsubota, K.; Takamizawa, T.; Kurokawa, H.; Rikuta, A.; Ando, S.; Miyazaki, M. Effect of metal conditioners on polymerization behavior of bonding agents. J. Oral Sci. 2005, 47, 171–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andersson, J.; Areva, S.; Lindén, M. Influences of material characteristics on ibuprofen drug loading and release profiles from ordered micro-and mesoporous silica matrices. Chem. Mater. 2004, 16, 4160–4167. [Google Scholar] [CrossRef]
- Can-Karabulut, D.C.; Oz, F.T.; Karabulut, B.; Batmaz, I.; Ilk, O. Adhesion to Primary and Permanent Dentin and a Simple Model Approach. Eur. J. Dent. 2009, 3, 32–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matos, A.B.; Trevelin, L.T.; Da Silva, B.T.F.; Francisconi-Dos-Rios, L.F.; Siriani, L.K.; Cardoso, M.V. Bonding efficiency and durability: Current possibilities. Braz. Oral Res. 2017, 31, e57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, S.R.; Vargas, M.A.; Chung, I.; Pashley, D.H.; Campbell, J.A.; Laffoon, J.E.; Qian, F. Resin-dentin interfacial ultrastructure and microtensile dentin bond strength after five-year water storage. Oper. Dent. 2005, 29, 705–712. [Google Scholar]
- Maman, P.; Nagpal, M.; Gilhotra, R.M.; Aggarwal, G. Nano Era of Dentistry—An Update. Curr. Drug Deliv. 2018, 15, 186–204. [Google Scholar] [CrossRef]
- Makvandi, P.; Gu, J.T.; Zare, E.N.; Ashtari, B.; Moeini, A.; Tay, F.R.; Niu, L.-N. Polymeric and inorganic nanoscopical antimicrobial fillers in dentistry. Acta Biomater. 2020, 101, 69–101. [Google Scholar] [CrossRef] [PubMed]
- Watson, T.F.; Atmeh, A.R.; Sajini, S.; Cook, R.J.; Festy, F. Present and future of glass-ionomers and calcium-silicate cements as bioactive materials in dentistry: Biophotonics-based interfacial analyses in health and disease. Dent. Mater. 2014, 30, 50–61. [Google Scholar] [CrossRef] [Green Version]
- Profeta, A.C. Dentine bonding agents comprising calcium-silicates to support proactive dental care: Origins, development and future. Dent. Mater. J. 2014, 33, 443–452. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.-H.; Um, C.-M.; Lee, I.-B. Rheological properties of resin composites according to variations in monomer and filler composition. Dent. Mater. 2006, 22, 515–526. [Google Scholar] [CrossRef] [PubMed]
- Rosa, A.; Da Silva, E.; Tostes, M. Scanning electron microscopy analysis of microstructure of the adhesive interface between resin and dentin treated with papain gel. Indian J. Dent. Res. 2015, 26, 77–81. [Google Scholar] [CrossRef]
- Anchieta, R.B.; Oliveira, F.G.; Sundfeld, R.H.; Rahal, V.; Machado, L.S.; Alexandre, R.S.; Sundefeld, M.L.M.M.; Rocha, E.P. Analysis of hybrid layer thickness, resin tag length and their correlation with microtensile bond strength using a total etch adhesive to intact dentin. Acta Odontol. Lat. 2011, 24, 272–278. [Google Scholar]
- Giordano, D.; Russell, J.K.; González-García, D.; Bersani, D.; Dingwell, D.B.; Negro, C.D. Raman Spectroscopy from Laboratory and Proximal to Remote Sensing: A Tool for the Volcanological Sciences. Remote Sens. 2020, 12, 805. [Google Scholar] [CrossRef] [Green Version]
- Akram, Z.; Aati, S.; Ngo, H.; Fawzy, A. pH-dependent delivery of chlorhexidine from PGA grafted mesoporous silica nanoparticles at resin-dentin interface. J. Nanobiotechnol. 2021, 19, 1–16. [Google Scholar] [CrossRef]
- Kasraei, S.H.; Atai, M.; Khamverdi, Z.; Nejad, S.K. Effect of Nanofiller Addition to an Experimental Dentin Adhesive on Microtensile Bond Strength to Human Dentin. Front. Dent. 2009, 6, 91–96. [Google Scholar]
- Kim, J.-S.; Cho, B.-H.; Lee, I.-B.; Um, C.-M.; Lim, B.-S.; Oh, M.-H.; Chang, C.-G.; Son, H.-H. Effect of the hydrophilic nanofiller loading on the mechanical properties and the microtensile bond strength of an ethanol-based one-bottle dentin adhesive. J. Biomed. Mater. Res. Part B Appl. Biomater. 2005, 72, 284–291. [Google Scholar] [CrossRef]
- Fallahzadeh, F.; Safarzadeh-Khosroshahi, S.; Atai, M. Dentin bonding agent with improved bond strength to dentin through incorporation of sepiolite nanoparticles. J. Clin. Exp. Dent. 2017, 9, e738–e742. [Google Scholar] [CrossRef] [Green Version]
- International Organization for Standardization. ISO/TS 11405 Dentistry—Testing of Adhesion to Tooth Structure, 3rd ed.; International Organization for Standardization: Geneva, Switzerland, 2015. [Google Scholar]
- Gale, M.; Darvell, B. Thermal cycling procedures for laboratory testing of dental restorations. J. Dent. 1999, 27, 89–99. [Google Scholar] [CrossRef]
- Helvatjoglu-Antoniades, M.; Koliniotou-Kubia, E.; Dionyssopoulos, P. The effect of thermal cycling on the bovine dentine shear bond strength of current adhesive systems. J. Oral Rehabil. 2004, 31, 911–917. [Google Scholar] [CrossRef] [PubMed]
- Aguiar, T.R.; De Oliveira, M.; Arrais, C.A.; Ambrosano, G.M.; Rueggeberg, F.; Giannini, M. The effect of photopolymerization on the degree of conversion, polymerization kinetic, biaxial flexure strength, and modulus of self-adhesive resin cements. J. Prosthet. Dent. 2015, 113, 128–134. [Google Scholar] [CrossRef]
- Leitune, V.C.B.; Collares, F.M.; Trommer, R.M.; Andrioli, D.G.; Bergmann, C.P.; Samuel, S.M.W. The addition of nanostructured hydroxyapatite to an experimental adhesive resin. J. Dent. 2013, 41, 321–327. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Wang, Y. Improved degree of conversion of model self-etching adhesives through their interaction with dentine. J. Dent. 2012, 40, 57–63. [Google Scholar] [CrossRef] [Green Version]






| μTBS (MPa) (Mean ± SD) | Failure Mode Analysis (%) | |||||
|---|---|---|---|---|---|---|
| Group (n = 10) | Non-Aged | Aged | p-Value * | Adhesive | Cohesive | Mixed |
| 1. Si-EA-0% (Control) | 24.62 ± 2.53 a A | - | <0.01 | 70 | 10 | 20 |
| - | 20.77 ± 2.08 a B | 100 | 0 | 0 | ||
| 2. Si-EA-5% | 28.85 ± 2.53 b A | 80 | 0 | 20 | ||
| - | 23.77 ± 2.49 a B | 100 | 0 | 0 | ||
| 3. Si-EA-10% | 34.36 ± 4.23 c A | 50 | 0 | 50 | ||
| - | 27.07 ± 2.49 b B | 80 | 10 | 10 | ||
| 4. Si-EA-15% | 36.17 ± 3.31 c A | 70 | 0 | 30 | ||
| - | 29.97 ± 4.18 b B | 100 | 0 | 0 | ||
| Groups | DC (Mean ± SD) | Tukey’s | |
|---|---|---|---|
| 1 | Si-EA-0% (UA-Control) | 48.2 ± 3.5 | A |
| 2 | Si-EA-5% | 47.4 ± 3.1 | A |
| 3 | Si-EA-10% | 41.9 ± 2.4 | B |
| 4 | Si-EA-15% | 37.6 ± 2.7 | B |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhenaki, A.M.; Attar, E.A.; Alshahrani, A.; Farooq, I.; Vohra, F.; Abduljabbar, T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers 2021, 13, 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071093
Alhenaki AM, Attar EA, Alshahrani A, Farooq I, Vohra F, Abduljabbar T. Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study. Polymers. 2021; 13(7):1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071093
Chicago/Turabian StyleAlhenaki, Aasem M., Esra A. Attar, Abdullah Alshahrani, Imran Farooq, Fahim Vohra, and Tariq Abduljabbar. 2021. "Dentin Bond Integrity of Filled and Unfilled Resin Adhesive Enhanced with Silica Nanoparticles—An SEM, EDX, Micro-Raman, FTIR and Micro-Tensile Bond Strength Study" Polymers 13, no. 7: 1093. https://0-doi-org.brum.beds.ac.uk/10.3390/polym13071093